Biomaterials-assisted spheroid engineering for regenerative therapy
- PMID: 34154700
- PMCID: PMC8328824
- DOI: 10.5483/BMBRep.2021.54.7.059
Biomaterials-assisted spheroid engineering for regenerative therapy
Abstract
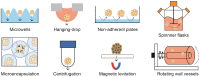
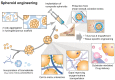
Cell-based therapy is a promising approach in the field of regenerative medicine. As cells are formed into spheroids, their survival, functions, and engraftment in the transplanted site are significantly improved compared to single cell transplantation. To improve the therapeutic effect of cell spheroids even further, various biomaterials (e.g., nano- or microparticles, fibers, and hydrogels) have been developed for spheroid engineering. These biomaterials not only can control the overall spheroid formation (e.g., size, shape, aggregation speed, and degree of compaction), but also can regulate cell-to-cell and cell-to-matrix interactions in spheroids. Therefore, cell spheroids in synergy with biomaterials have recently emerged for cell-based regenerative therapy. Biomaterials-assisted spheroid engineering has been extensively studied for regeneration of bone or/and cartilage defects, critical limb ischemia, and myocardial infarction. Furthermore, it has been expanded to pancreas islets and hair follicle transplantation. This paper comprehensively reviews biomaterials-assisted spheroid engineering for regenerative therapy. [BMB Reports 2021; 54(7): 356-367].
Conflict of interest statement
The authors have no conflicting interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

