Wnt Signaling: From Mesenchymal Cell Fate to Lipogenesis and Other Mature Adipocyte Functions
- PMID: 34155042
- PMCID: PMC8336005
- DOI: 10.2337/dbi20-0015
Wnt Signaling: From Mesenchymal Cell Fate to Lipogenesis and Other Mature Adipocyte Functions
Abstract
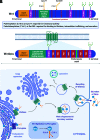
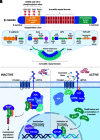
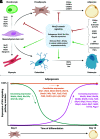
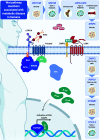
Wnt signaling is an ancient and evolutionarily conserved pathway with fundamental roles in the development of adipose tissues. Roles of this pathway in mesenchymal stem cell fate determination and differentiation have been extensively studied. Indeed, canonical Wnt signaling is a significant endogenous inhibitor of adipogenesis and promoter of other cell fates, including osteogenesis, chondrogenesis, and myogenesis. However, emerging genetic evidence in both humans and mice suggests central roles for Wnt signaling in body fat distribution, obesity, and metabolic dysfunction. Herein, we highlight recent studies that have begun to unravel the contributions of various Wnt pathway members to critical adipocyte functions, including carbohydrate and lipid metabolism. We further explore compelling evidence of complex and coordinated interactions between adipocytes and other cell types within adipose tissues, including stromal, immune, and endothelial cells. Given the evolutionary conservation and ubiquitous cellular distribution of this pathway, uncovering the contributions of Wnt signaling to cell metabolism has exciting implications for therapeutic intervention in widespread pathologic states, including obesity, diabetes, and cancers.
© 2021 by the American Diabetes Association.
Figures

References
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 - PubMed
-
- Loh KM, van Amerongen R, Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell 2016;38:643–655 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

