Dissecting and modeling photic and melanopsin effects to predict sleep disturbances induced by irregular light exposure in mice
- PMID: 34155139
- PMCID: PMC8237663
- DOI: 10.1073/pnas.2017364118
Dissecting and modeling photic and melanopsin effects to predict sleep disturbances induced by irregular light exposure in mice
Abstract
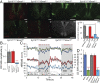
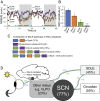
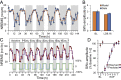
Artificial lighting, day-length changes, shift work, and transmeridian travel all lead to sleep-wake disturbances. The nychthemeral sleep-wake cycle (SWc) is known to be controlled by output from the central circadian clock in the suprachiasmatic nuclei (SCN), which is entrained to the light-dark cycle. Additionally, via intrinsically photosensitive retinal ganglion cells containing the photopigment melanopsin (Opn4), short-term light-dark alternations exert direct and acute influences on sleep and waking. However, the extent to which longer exposures typically experienced across the 24-h day exert such an effect has never been clarified or quantified, as disentangling sustained direct light effects (SDLE) from circadian effects is difficult. Recording sleep in mice lacking a circadian pacemaker, either through transgenesis (Syt10cre/creBmal1fl/- ) or SCN lesioning and/or melanopsin-based phototransduction (Opn4-/- ), we uncovered, contrary to prevailing assumptions, that the contribution of SDLE is as important as circadian-driven input in determining SWc amplitude. Specifically, SDLE were primarily mediated (>80%) through melanopsin, of which half were then relayed through the SCN, revealing an ancillary purpose for this structure, independent of its clock function in organizing SWc. Based on these findings, we designed a model to estimate the effect of atypical light-dark cycles on SWc. This model predicted SWc amplitude in mice exposed to simulated transequatorial or transmeridian paradigms. Taken together, we demonstrate this SDLE is a crucial mechanism influencing behavior on par with the circadian system. In a broader context, these findings mandate considering SDLE, in addition to circadian drive, for coping with health consequences of atypical light exposure in our society.
Keywords: circadian and noncircadian; melanopsin; photoperiods; phototransduction; sleep–wake cycle.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Bedrosian T. A., Nelson R. J., Influence of the modern light environment on mood. Mol. Psychiatry 18, 751–757 (2013). - PubMed
-
- Stephenson K. M., Schroder C. M., Bertschy G., Bourgin P., Complex interaction of circadian and non-circadian effects of light on mood: Shedding new light on an old story. Sleep Med. Rev. 16, 445–454 (2012). - PubMed
-
- Vandewalle G., Maquet P., Dijk D. J., Light as a modulator of cognitive brain function. Trends Cogn. Sci. 13, 429–438 (2009). - PubMed
-
- Kilic U., Hubbard J., Bourgin P., “Circadian rhythm sleep disorders, 5. Treatment” in Sleep Medicine Textbook, Bassetti C., Dogas Z., Peigneux P., Eds. (European Sleep Research Society, 2014), pp. 357–368.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

