Impact of early response on outcomes in AL amyloidosis following treatment with frontline Bortezomib
- PMID: 34155191
- PMCID: PMC8217489
- DOI: 10.1038/s41408-021-00510-7
Impact of early response on outcomes in AL amyloidosis following treatment with frontline Bortezomib
Abstract
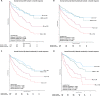
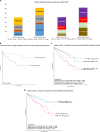
The outcomes in systemic AL amyloidosis are dependent on the depth of haematologic response. However, there is limited data on the impact of the speed of response on outcomes. Here we report the impact of speed of response in a cohort of AL patients treated with upfront Bortezomib. Patients seen from February 2010 until August 2019 are included in the present analysis. 1194 & 1133 patients comprised the ITT and 1-month landmark cohorts. In the landmark cohort, 137 (11.5%), 270 (22.6%), 252 (21.1%) and 352 (31.1%) patients had a CR, VGPR, PR and NR at 1-month. Patients with ≥ VGPR at 1-month had significantly better survival (median not reached; at the end of 1, 2, 5,10 years, 87%/92%, 83%/87%, 68%/72% and 63%/58% of patients in CR/VGPR, respectively, were alive) compared to those with a PR (median OS 60 months) or NR (median OS 32 months) (p < 0.005). At 1-month, patients with CR and iFLC < 20 mg/l had a significantly better survival compared to CR and iFLC > 20 mg/l (p = 0.005). Reaching ≥ VGPR at 1-month significantly improved survival in all Mayo disease stages. In conclusion, patients achieving an early deep haematologic response have a significantly superior survival irrespective of cardiac involvement.
Conflict of interest statement
A.D.W. has received honoraria from Janssen, GSK, Celgene, and Takeda. The other authors do not have any conflict of interest to disclose.
Figures


References
-
- Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J. Clin. Oncol. 2012;30:4541–9. doi: 10.1200/JCO.2011.37.7614. - DOI - PubMed
-
- Godara A, Toskic D, Rosenthal B, Varga C, Kugelmass A, Zhou P, et al. In systemic light-chain amyloidosis complete and very good partial responses are not enough: involved free light chain (iFLC) Levels < 10mg/L are associated with optimal long-term survival. Blood. 2019;134:4369. doi: 10.1182/blood-2019-127004. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

