Iron and sulfate reduction structure microbial communities in (sub-)Antarctic sediments
- PMID: 34155335
- PMCID: PMC8630232
- DOI: 10.1038/s41396-021-01014-9
Iron and sulfate reduction structure microbial communities in (sub-)Antarctic sediments
Abstract
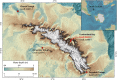
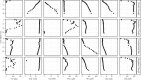
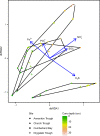
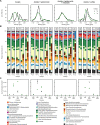
Permanently cold marine sediments are heavily influenced by increased input of iron as a result of accelerated glacial melt, weathering, and erosion. The impact of such environmental changes on microbial communities in coastal sediments is poorly understood. We investigated geochemical parameters that shape microbial community compositions in anoxic surface sediments of four geochemically differing sites (Annenkov Trough, Church Trough, Cumberland Bay, Drygalski Trough) around South Georgia, Southern Ocean. Sulfate reduction prevails in Church Trough and iron reduction at the other sites, correlating with differing local microbial communities. Within the order Desulfuromonadales, the family Sva1033, not previously recognized for being capable of dissimilatory iron reduction, was detected at rather high relative abundances (up to 5%) while other members of Desulfuromonadales were less abundant (<0.6%). We propose that Sva1033 is capable of performing dissimilatory iron reduction in sediment incubations based on RNA stable isotope probing. Sulfate reducers, who maintain a high relative abundance of up to 30% of bacterial 16S rRNA genes at the iron reduction sites, were also active during iron reduction in the incubations. Thus, concurrent sulfate reduction is possibly masked by cryptic sulfur cycling, i.e., reoxidation or precipitation of produced sulfide at a small or undetectable pool size. Our results show the importance of iron and sulfate reduction, indicated by ferrous iron and sulfide, as processes that shape microbial communities and provide evidence for one of Sva1033's metabolic capabilities in permanently cold marine sediments.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- D’Hondt S, Jørgensen BB, Miller DJ, Batzke A, Blake R, Cragg BA, et al. Distributions of microbial activities in deep subseafloor sediments. Science. 2004;306:2216–21. - PubMed
-
- Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR, Cullen D, et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta. 1979;43:1075–90.
-
- Parkes RJ, Cragg B, Roussel E, Webster G, Weightman A, Sass H. A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere: geosphere interactions. Mar Geol. 2014;352:409–25.
-
- Arnosti C. Microbial extracellular enzymes and the marine carbon cycle. Annu Rev Mar Sci. 2011;3:401–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

