Media and strain studies for the scaled production of cis-enone resorcylic acid lactones as feedstocks for semisynthesis
- PMID: 34155352
- PMCID: PMC8313427
- DOI: 10.1038/s41429-021-00432-3
Media and strain studies for the scaled production of cis-enone resorcylic acid lactones as feedstocks for semisynthesis
Abstract
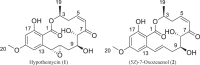
Resorcylic acid lactones (RALs) with a cis-enone moiety, represented by hypothemycin (1) and (5Z)-7-oxozeaenol (2), are fungal secondary metabolites with irreversible inhibitory activity against protein kinases, with particularly selective activity for inhibition of TAK1 (transforming growth factor beta-activated kinase 1). Gram-scale quantities of these compounds were needed as feedstock for semi-synthesizing RAL-analogues in a step-economical fashion. To do so, this study had three primary goals: identifying fungi that biosynthesized 1 and 2, enhancing their production by optimizing the fermentation conditions on the lab scale, and developing straight forward purification processes. After evaluating 536 fungal extracts via an in-house dereplication protocol, three strains were identified as producing cis-enone RALs (i.e., MSX78495, MSX63935, MSX45109). Screening these fungal strains on three grain-based media revealed enhanced production of 1 by strain MSX78495 on oatmeal medium, while rice medium increased the biosynthesis of 2 by strain MSX63935. Furthermore, the purification processes were improved, moving away from HPLC purification to utilizing two to four cycles of resuspension and centrifugation in small volumes of organic solvents, generating gram-scale quantities of these metabolites readily. In addition, studying the chemistry profiles of strains MSX78495 and MSX63935 resulted in the isolation of ten other RALs (3-12), two radicinin analogues (13-14), and six benzopyranones (15-20), with 19 and 20 being newly described chlorinated benzopyranones.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interest(s): NHO is a member of the Scientific Advisory Board of Mycosynthetix, Inc.
Figures



Similar articles
-
Cis-enone resorcylic acid lactones (RALs) as irreversible protein kinase inhibitors.Curr Pharm Des. 2012;18(9):1186-98. doi: 10.2174/138161212799436395. Curr Pharm Des. 2012. PMID: 22316158 Review.
-
Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides.Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4234-9. doi: 10.1073/pnas.0600445103. Epub 2006 Mar 6. Proc Natl Acad Sci U S A. 2006. PMID: 16537514 Free PMC article.
-
Divergent syntheses of resorcylic acid lactones: L-783277, LL-Z1640-2, and hypothemycin.Chemistry. 2009 Nov 2;15(43):11490-7. doi: 10.1002/chem.200901373. Chemistry. 2009. PMID: 19821460
-
Beta resorcylic acid lactones (RALs) from fungi: chemistry, biology, and biosynthesis.Arch Pharm Res. 2020 Nov;43(11):1093-1113. doi: 10.1007/s12272-020-01275-6. Epub 2020 Oct 28. Arch Pharm Res. 2020. PMID: 33113097 Review.
-
Resorcylic acid lactones with cytotoxic and NF-κB inhibitory activities and their structure-activity relationships.J Nat Prod. 2011 May 27;74(5):1126-31. doi: 10.1021/np200062x. Epub 2011 Apr 22. J Nat Prod. 2011. PMID: 21513293 Free PMC article.
Cited by
-
Semisynthesis of Hypothemycin Analogues Targeting the C8-C9 Diol.J Nat Prod. 2022 Aug 26;85(8):2018-2025. doi: 10.1021/acs.jnatprod.2c00434. Epub 2022 Jul 14. J Nat Prod. 2022. PMID: 35834411 Free PMC article. Review.
-
Studies on the epipolythiodioxopiperazine alkaloid verticillin D: Scaled production, streamlined purification, and absolute configuration.Phytochemistry. 2025 Aug;236:114492. doi: 10.1016/j.phytochem.2025.114492. Epub 2025 Mar 25. Phytochemistry. 2025. PMID: 40147592 Free PMC article.
-
Bioactivity-driven fungal metabologenomics identifies antiproliferative stemphone analogs and their biosynthetic gene cluster.Metabolomics. 2024 Aug 2;20(5):90. doi: 10.1007/s11306-024-02153-8. Metabolomics. 2024. PMID: 39095664 Free PMC article.
-
Dereplication of Fungal Metabolites by NMR-Based Compound Networking Using MADByTE.J Nat Prod. 2022 Mar 25;85(3):614-624. doi: 10.1021/acs.jnatprod.1c00841. Epub 2022 Jan 12. J Nat Prod. 2022. PMID: 35020372 Free PMC article.
-
Discovery of Anticancer Agents of Diverse Natural Origin.J Nat Prod. 2022 Mar 25;85(3):702-719. doi: 10.1021/acs.jnatprod.2c00036. Epub 2022 Feb 25. J Nat Prod. 2022. PMID: 35213158 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

