Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration
- PMID: 34155382
- PMCID: PMC7611697
- DOI: 10.1038/s41556-021-00699-6
Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration
Abstract
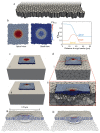
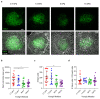
Intestinal organoids capture essential features of the intestinal epithelium such as crypt folding, cellular compartmentalization and collective movements. Each of these processes and their coordination require patterned forces that are at present unknown. Here we map three-dimensional cellular forces in mouse intestinal organoids grown on soft hydrogels. We show that these organoids exhibit a non-monotonic stress distribution that defines mechanical and functional compartments. The stem cell compartment pushes the extracellular matrix and folds through apical constriction, whereas the transit amplifying zone pulls the extracellular matrix and elongates through basal constriction. The size of the stem cell compartment depends on the extracellular-matrix stiffness and endogenous cellular forces. Computational modelling reveals that crypt shape and force distribution rely on cell surface tensions following cortical actomyosin density. Finally, cells are pulled out of the crypt along a gradient of increasing tension. Our study unveils how patterned forces enable compartmentalization, folding and collective migration in the intestinal epithelium.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures














Comment in
-
The mechanics of crypt morphogenesis.Nat Cell Biol. 2021 Jul;23(7):678-679. doi: 10.1038/s41556-021-00703-z. Nat Cell Biol. 2021. PMID: 34155380 No abstract available.
References
-
- Chacón-Martínez CA, Koester J, Wickström SA. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 2018;145:dev165399. - PubMed
-
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

