Predictable control of RNA lifetime using engineered degradation-tuning RNAs
- PMID: 34155402
- PMCID: PMC8238901
- DOI: 10.1038/s41589-021-00816-4
Predictable control of RNA lifetime using engineered degradation-tuning RNAs
Abstract
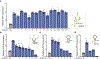
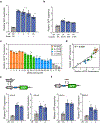
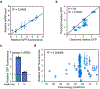
The ability to tune RNA and gene expression dynamics is greatly needed for biotechnological applications. Native RNA stabilizers or engineered 5' stability hairpins have been used to regulate transcript half-life to control recombinant protein expression. However, these methods have been mostly ad hoc and hence lack predictability and modularity. Here, we report a library of RNA modules called degradation-tuning RNAs (dtRNAs) that can increase or decrease transcript stability in vivo and in vitro. dtRNAs enable modulation of transcript stability over a 40-fold dynamic range in Escherichia coli with minimal influence on translation initiation. We harness dtRNAs in messenger RNAs and noncoding RNAs to tune gene circuit dynamics and enhance CRISPR interference in vivo. Use of stabilizing dtRNAs in cell-free transcription-translation reactions also tunes gene and RNA aptamer production. Finally, we combine dtRNAs with toehold switch sensors to enhance the performance of paper-based norovirus diagnostics, illustrating the potential of dtRNAs for biotechnological applications.
Conflict of interest statement
Competing interests
The authors declare the following competing financial interest(s): a patent application has been filed relating to this work.
Figures
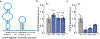






Similar articles
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Chemical Strategies to Modulate and Manipulate RNA Epigenetic Modifications.Acc Chem Res. 2025 Jun 3;58(11):1727-1741. doi: 10.1021/acs.accounts.4c00844. Epub 2025 Mar 18. Acc Chem Res. 2025. PMID: 40100209 Review.
-
Determining the Specificity of Cascade Binding, Interference, and Primed Adaptation In Vivo in the Escherichia coli Type I-E CRISPR-Cas System.mBio. 2018 Apr 17;9(2):e02100-17. doi: 10.1128/mBio.02100-17. mBio. 2018. PMID: 29666291 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Automated devices for identifying peripheral arterial disease in people with leg ulceration: an evidence synthesis and cost-effectiveness analysis.Health Technol Assess. 2024 Aug;28(37):1-158. doi: 10.3310/TWCG3912. Health Technol Assess. 2024. PMID: 39186036 Free PMC article.
Cited by
-
Conditional RNA interference in mammalian cells via RNA transactivation.Nat Commun. 2024 Aug 10;15(1):6855. doi: 10.1038/s41467-024-50600-w. Nat Commun. 2024. PMID: 39127751 Free PMC article.
-
Bioinformatic Assessment of Factors Affecting the Correlation between Protein Abundance and Elongation Efficiency in Prokaryotes.Int J Mol Sci. 2022 Oct 9;23(19):11996. doi: 10.3390/ijms231911996. Int J Mol Sci. 2022. PMID: 36233299 Free PMC article.
-
Bifunctional optogenetic switch for improving shikimic acid production in E. coli.Biotechnol Biofuels Bioprod. 2022 Feb 7;15(1):13. doi: 10.1186/s13068-022-02111-3. Biotechnol Biofuels Bioprod. 2022. PMID: 35418155 Free PMC article.
-
Cellular Computational Logic Using Toehold Switches.Int J Mol Sci. 2022 Apr 12;23(8):4265. doi: 10.3390/ijms23084265. Int J Mol Sci. 2022. PMID: 35457085 Free PMC article.
-
Cotranscriptionally encoded RNA strand displacement circuits.Sci Adv. 2022 Mar 25;8(12):eabl4354. doi: 10.1126/sciadv.abl4354. Epub 2022 Mar 23. Sci Adv. 2022. PMID: 35319994 Free PMC article.
References
-
- Cambray G, Guimaraes JC & Arkin AP Evaluation of 244,000 synthetic sequences reveals design principles to optimize translation in Escherichia coli. Nat Biotechnol 36, 1005–1015 (2018). - PubMed
-
- Delivering the promise of RNA therapeutics. Nat Med 25, 1321–1321 (2019). - PubMed
-
- Pardee K et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 165, 1255–1266 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

