A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease
- PMID: 34155411
- PMCID: PMC8988051
- DOI: 10.1038/s41591-021-01369-8
A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease
Abstract
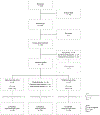
Dominantly inherited Alzheimer's disease (DIAD) causes predictable biological changes decades before the onset of clinical symptoms, enabling testing of interventions in the asymptomatic and symptomatic stages to delay or slow disease progression. We conducted a randomized, placebo-controlled, multi-arm trial of gantenerumab or solanezumab in participants with DIAD across asymptomatic and symptomatic disease stages. Mutation carriers were assigned 3:1 to either drug or placebo and received treatment for 4-7 years. The primary outcome was a cognitive end point; secondary outcomes included clinical, cognitive, imaging and fluid biomarker measures. Fifty-two participants carrying a mutation were assigned to receive gantenerumab, 52 solanezumab and 40 placebo. Both drugs engaged their Aβ targets but neither demonstrated a beneficial effect on cognitive measures compared to controls. The solanezumab-treated group showed a greater cognitive decline on some measures and did not show benefits on downstream biomarkers. Gantenerumab significantly reduced amyloid plaques, cerebrospinal fluid total tau, and phospho-tau181 and attenuated increases of neurofilament light chain. Amyloid-related imaging abnormalities edema was observed in 19.2% (3 out of 11 were mildly symptomatic) of the gantenerumab group, 2.5% of the placebo group and 0% of the solanezumab group. Gantenerumab and solanezumab did not slow cognitive decline in symptomatic DIAD. The asymptomatic groups showed no cognitive decline; symptomatic participants had declined before reaching the target doses.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures


References
-
- Braak H, Thal DR, Ghebremedhin E & Del Tredici K Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- R01AG046179, R01AG053267-S1/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P01 AG026276/AG/NIA NIH HHS/United States
- R01 AG046179/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R01 AG053267/AG/NIA NIH HHS/United States
- UF1 AG032438/AG/NIA NIH HHS/United States
- U01AG042791/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- U19 AG032438/AG/NIA NIH HHS/United States
- U01 AG042791/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- U19AG032438/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- S10 OD025214/OD/NIH HHS/United States
- U01AG042791-S1/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)

