A Wavelet-Based Approach for Estimating Time-Varying Connectivity in Resting-State Functional Magnetic Resonance Imaging
- PMID: 34155908
- PMCID: PMC9271336
- DOI: 10.1089/brain.2021.0015
A Wavelet-Based Approach for Estimating Time-Varying Connectivity in Resting-State Functional Magnetic Resonance Imaging
Abstract
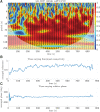
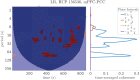
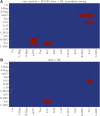
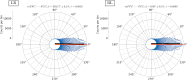
Introduction: The selection of an appropriate window size, window function, and functional connectivity (FC) metric in the sliding window method is not straightforward due to the absence of ground truth. Methods: A previously proposed wavelet-based method was accordingly adjusted for estimating time-varying FC (TVFC) and was applied to a large high-quality, low-motion dataset of 400 resting-state functional magnetic resonance imaging data. Specifically, the wavelet coherence magnitude and relative phase were averaged across wavelet (frequency) scales to yield TVFC and synchronization patterns. To assess whether the observed fluctuations in TVFC were statistically significant (dynamic FC [dFC]; the distinction between TVFC and dFC is intentional), surrogate data were generated using the multivariate phase randomization (MVPR) and multivariate autoregressive randomization (MVAR) methods to define the null hypothesis of dFC absence. Results: By averaging across all frequencies, core regions of the default mode network (DMN; medial prefrontal and posterior cingulate cortices, inferior parietal lobes, hippocampal formation) were found to exhibit dFC (test-retest reproducibility of 90%) and were also synchronized in activity (-15° ≤ phase ≤15°). When averaging across distinct frequency bands, the same dynamic connections were identified, with the majority of them identified in the frequency range (0.01, 0.198) Hz, though with lower test-retest reproducibility (<66%). Additional analysis suggested that MVPR method better preserved properties (p < 10-10), including time-averaged coherence, of the original data compared with MVAR approach. Conclusions: The wavelet-based approach identified dynamic associations between the core DMN regions with fewer choices in parameters, compared with sliding window method. Impact statement We employed a wavelet-based method, previously used in the literature, and proposed modifications to assess time-varying functional connectivity in resting-state functional magnetic resonance imaging. With this approach, dynamic connections within the default mode network were identified, involving the medial prefrontal and posterior cingulate cortices, inferior parietal lobes, and hippocampal formation, which were also highly consistent in test-retest analysis (test-retest reproducibility of 90%), without the need to select window size, window function, and functional connectivity metric as with the sliding window method, whereby no consensus on the appropriate choices of hyperparameters currently exists in the literature.
Keywords: Morlet wavelet; dynamic functional connectivity; multivariate autoregressive randomization; multivariate phase randomization; surrogate data; wavelet transform coherence.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Berens P. 2009. CircStat: AMATLABToolbox for circular statistics. J Stat Softw 31:21.
-
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

