Mechanistic underpinning of an inside-out concept for autoimmunity in multiple sclerosis
- PMID: 34156169
- PMCID: PMC8351380
- DOI: 10.1002/acn3.51401
Mechanistic underpinning of an inside-out concept for autoimmunity in multiple sclerosis
Abstract
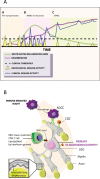
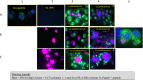
The neuroinflammatory disease multiple sclerosis is driven by autoimmune pathology in the central nervous system. However, the trigger of the autoimmune pathogenic process is unknown. MS models in immunologically naïve, specific-pathogen-free bred rodents support an exogenous trigger, such as an infection. The validity of this outside-in pathogenic concept for MS has been frequently challenged by the difficulty to translate pathogenic concepts developed in these models into effective therapies for the MS patient. Studies in well-validated non-human primate multiple sclerosis models where, just like in humans, the autoimmune pathogenic process develops from an experienced immune system trained by prior infections, rather support an endogenous trigger. Data reviewed here corroborate the validity of this inside-out pathogenic concept for multiple sclerosis. They also provide a plausible sequence of events reminiscent of Wilkin's primary lesion theory: (i) that autoimmunity is a physiological response of the immune system against excess antigen turnover in diseased tissue (the primary lesion) and (ii) that individuals developing autoimmune disease are (genetically predisposed) high responders against critical antigens. Data obtained in multiple sclerosis brains reveal the presence in normally appearing white matter of myelinated axons where myelin sheaths have locally dissociated from their enwrapped axon (i.e., blistering). The ensuing disintegration of axon-myelin units potentially causes the excess systemic release of post-translationally modified myelin. Data obtained in a unique primate multiple sclerosis model revealed a core pathogenic role of T cells present in the normal repertoire, which hyper-react to post-translationally modified (citrullinated) myelin-oligodendrocyte glycoprotein and evoke clinical and pathological aspects of multiple sclerosis.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
No disclosures relevant to the publication are reported.
Figures

References
-
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol 2003;21:139–176. - PubMed
-
- Sercarz EE, Lehmann PV, Ametani A, et al. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol 1993;11:729–766. - PubMed
-
- Serre L, Girard M, Ramadan A, et al. Thymic‐specific serine protease limits central tolerance and exacerbates experimental autoimmune encephalomyelitis. J Immunol 2017;199:3748–3756. - PubMed
-
- ’t Hart BA, Gran B, Weissert R. EAE: imperfect but useful models of multiple sclerosis. Trends Mol Med 2011;17:119–125. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

