3D Photothermal Cryogels for Solar-Driven Desalination
- PMID: 34156821
- PMCID: PMC8289246
- DOI: 10.1021/acsami.1c05087
3D Photothermal Cryogels for Solar-Driven Desalination
Abstract
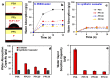
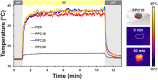
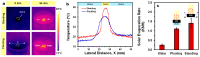
This paper reports the fabrication of photothermal cryogels for freshwater production via the solar-driven evaporation of seawater. Photothermal cryogels were prepared via in situ oxidative polymerization of pyrrole with ammonium persulfate on preformed poly(sodium acrylate) (PSA) cryogels. We found that the pyrrole concentration used in the fabrication process has a significant effect on the final PSA/PPy cryogels (PPCs), causing the as-formed polypyrrole (PPy) layer on the PPC to evolve from nanoparticles to lamellar sheets and to consolidated thin films. PPC fabricated using the lowest pyrrole concentration (i.e., PPC10) displays the best solar-evaporation efficiency compared to the other samples, which is further improved by switching the operative mode from floating to standing. Specifically, in the latter case, the apparent solar evaporation rate and solar-to-vapor conversion efficiency reach 1.41 kg m-2 h-1 and 96.9%, respectively, due to the contribution of evaporation from the exposed lateral surfaces. The distillate obtained from the condensed vapor, generated via solar evaporation of a synthetic seawater through PPC10, shows an at least 99.99% reduction of Na while all the other elements are reduced to a subppm level. We attribute the superior solar evaporation and desalination performance of PPC10 to its (i) higher photoabsorption efficiency, (ii) higher heat localization effect, (iii) open porous structure that facilitates vapor removal, (iv) rough pore surface that increases the surface area for light absorption and water evaporation, and (v) higher water-absorption capacity to ensure efficient water replenishment to the evaporative sites. It is anticipated that the gained know-how from this study would offer insightful guidelines to better designs of polymer-based 3D photothermal materials for solar evaporation as well as for other emerging solar-related applications.
Keywords: interfacial solar evaporation; poly(sodium acrylate); polypyrrole; porous hydrogels; solar−thermal conversion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- 2030 Water Resources Group . Charting Our Water Future; 2009; pp 1–185.
-
- Transforming Our World: The 2030 Agenda for Sustainable Development. In A New Era in Global Health; Rosa W., Ed.; Springer Publishing Company: New York, NY, 2017; pp 1–38.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

