Acoustic Mist Ionization Mass Spectrometry for Ultrahigh-Throughput Metabolomics Screening
- PMID: 34156839
- PMCID: PMC8264826
- DOI: 10.1021/acs.analchem.1c01616
Acoustic Mist Ionization Mass Spectrometry for Ultrahigh-Throughput Metabolomics Screening
Abstract
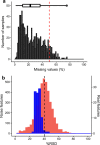
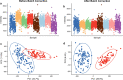
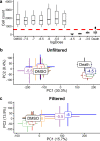
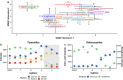
Incorporating safety data early in the drug discovery pipeline is key to reducing costly lead candidate failures. For a single drug development project, we estimate that several thousand samples per day require screening (<10 s per acquisition). While chromatography-based metabolomics has proven value at predicting toxicity from metabolic biomarker profiles, it lacks sufficiently high sample throughput. Acoustic mist ionization mass spectrometry (AMI-MS) is an atmospheric pressure ionization approach that can measure metabolites directly from 384-well plates with unparalleled speed. We sought to implement a signal processing and data analysis workflow to produce high-quality AMI-MS metabolomics data and to demonstrate its application to drug safety screening. An existing direct infusion mass spectrometry workflow was adapted, extended, optimized, and tested, utilizing three AMI-MS data sets acquired from technical and biological replicates of metabolite standards and HepG2 cell lysates and a toxicity study. Driven by criteria to minimize variance and maximize feature counts, an algorithm to extract the pulsed scan data was designed; parameters for signal-to-noise-ratio, replicate filter, sample filter, missing value filter, and RSD filter were all optimized; normalization and batch correction strategies were adapted; and cell phenotype filtering was implemented to exclude high cytotoxicity samples. The workflow was demonstrated using a highly replicated HepG2 toxicity data set, comprising 2772 samples from exposures to 16 drugs across 9 concentrations and generated in under 5 h, revealing metabolic phenotypes and individual metabolite changes that characterize specific modes of action. This AMI-MS workflow opens the door to ultrahigh-throughput metabolomics screening, increasing the rate of sample analysis by approximately 2 orders of magnitude.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Morgan P.; Brown D. G.; Lennard S.; Anderton M. J.; Barrett J. C.; Eriksson U.; Fidock M.; Hamrén B.; Johnson A.; March R. E.; Matcham J.; Mettetal J.; Nicholls D. J.; Platz S.; Rees S.; Snowden M. A.; Pangalos M. N. Impact of a Five-Dimensional Framework on R&D Productivity at AstraZeneca. Nat. Rev. Drug Discovery 2018, 17, 167–181. 10.1038/nrd.2017.244. - DOI - PubMed
-
- Sinclair I.; Bachman M.; Addison D.; Rohman M.; Murray D. C.; Davies G.; Mouchet E.; Tonge M. E.; Stearns R. G.; Ghislain L.; Datwani S. S.; Majlof L.; Hall E.; Jones G. R.; Hoyes E.; Olechno J.; Ellson R. N.; Barran P. E.; Pringle S. D. Acoustic Mist Ionization Platform for Direct and Contactless Ultrahigh-Throughput Mass Spectrometry Analysis of Liquid Samples. Anal. Chem. 2019, 91, 3790–3794. 10.1021/acs.analchem.9b00142. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

