The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells
- PMID: 34156871
- PMCID: PMC8384477
- DOI: 10.1152/ajplung.00223.2021
The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells
Abstract
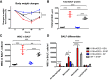
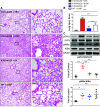
Acute lung injury (ALI) leading to acute respiratory distress syndrome is the major cause of COVID-19 lethality. Cell entry of SARS-CoV-2 occurs via the interaction between its surface spike protein (SP) and angiotensin-converting enzyme-2 (ACE2). It is unknown if the viral spike protein alone is capable of altering lung vascular permeability in the lungs or producing lung injury in vivo. To that end, we intratracheally instilled the S1 subunit of SARS-CoV-2 spike protein (S1SP) in K18-hACE2 transgenic mice that overexpress human ACE2 and examined signs of COVID-19-associated lung injury 72 h later. Controls included K18-hACE2 mice that received saline or the intact SP and wild-type (WT) mice that received S1SP. K18-hACE2 mice instilled with S1SP exhibited a decline in body weight, dramatically increased white blood cells and protein concentrations in bronchoalveolar lavage fluid (BALF), upregulation of multiple inflammatory cytokines in BALF and serum, histological evidence of lung injury, and activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways in the lung. K18-hACE2 mice that received either saline or SP exhibited little or no evidence of lung injury. WT mice that received S1SP exhibited a milder form of COVID-19 symptoms, compared with the K18-hACE2 mice. Furthermore, S1SP, but not SP, decreased cultured human pulmonary microvascular transendothelial resistance (TER) and barrier function. This is the first demonstration of a COVID-19-like response by an essential virus-encoded protein by SARS-CoV-2 in vivo. This model of COVID-19-induced ALI may assist in the investigation of new therapeutic approaches for the management of COVID-19 and other coronaviruses.
Keywords: COVID-19 murine model; SARS-CoV-2; acute lung injury; endothelial permeability; spike protein.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Acute lung injury induced by recombinant SARS-CoV-2 spike protein subunit S1 in mice.Respir Res. 2025 Feb 19;26(1):59. doi: 10.1186/s12931-025-03143-7. Respir Res. 2025. PMID: 39972348 Free PMC article.
-
Alcohol Increases Lung Angiotensin-Converting Enzyme 2 Expression and Exacerbates Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Subunit 1-Induced Acute Lung Injury in K18-hACE2 Transgenic Mice.Am J Pathol. 2022 Jul;192(7):990-1000. doi: 10.1016/j.ajpath.2022.03.012. Epub 2022 Apr 25. Am J Pathol. 2022. PMID: 35483427 Free PMC article.
-
A human-ACE2 knock-in mouse model for SARS-CoV-2 infection recapitulates respiratory disorders but avoids neurological disease associated with the transgenic K18-hACE2 model.mBio. 2025 May 14;16(5):e0072025. doi: 10.1128/mbio.00720-25. Epub 2025 Apr 24. mBio. 2025. PMID: 40272151 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
Cited by
-
Targeting spike protein-induced TLR/NET axis by COVID-19 therapeutic NRICM102 ameliorates pulmonary embolism and fibrosis.Pharmacol Res. 2022 Oct;184:106424. doi: 10.1016/j.phrs.2022.106424. Epub 2022 Sep 5. Pharmacol Res. 2022. PMID: 36064077 Free PMC article.
-
SARS-CoV-2 Spike Protein and Mouse Coronavirus Inhibit Biofilm Formation by Streptococcus pneumoniae and Staphylococcus aureus.Int J Mol Sci. 2022 Mar 18;23(6):3291. doi: 10.3390/ijms23063291. Int J Mol Sci. 2022. PMID: 35328711 Free PMC article.
-
The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication.Signal Transduct Target Ther. 2022 Feb 23;7(1):57. doi: 10.1038/s41392-022-00907-1. Signal Transduct Target Ther. 2022. PMID: 35197452 Free PMC article. Review.
-
COVID-19 Vaccination and Alcohol Consumption: Justification of Risks.Pathogens. 2023 Jan 19;12(2):163. doi: 10.3390/pathogens12020163. Pathogens. 2023. PMID: 36839435 Free PMC article. Review.
-
SARS-CoV-2 Spike Protein and Its Receptor Binding Domain Promote a Proinflammatory Activation Profile on Human Dendritic Cells.Cells. 2021 Nov 23;10(12):3279. doi: 10.3390/cells10123279. Cells. 2021. PMID: 34943787 Free PMC article.
References
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi:10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ; RECOVERY Collaborative Group. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report (Preprint). medRxiv, 2020. doi:10.1101/2020.06.22.20137273. - DOI
-
- Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585: 268–272, 2020. doi:10.1038/s41586-020-2324-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

