The clear cell sarcoma functional genomic landscape
- PMID: 34156976
- PMCID: PMC8321568
- DOI: 10.1172/JCI146301
The clear cell sarcoma functional genomic landscape
Abstract
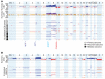
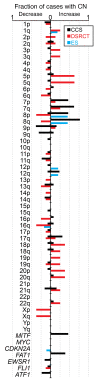
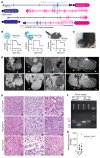
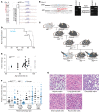
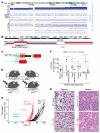
Clear cell sarcoma (CCS) is a deadly malignancy affecting adolescents and young adults. It is characterized by reciprocal translocations resulting in expression of the chimeric EWSR1-ATF1 or EWSR1-CREB1 fusion proteins, driving sarcomagenesis. Besides these characteristics, CCS has remained genomically uncharacterized. Copy number analysis of human CCSs showed frequent amplifications of the MITF locus and chromosomes 7 and 8. Few alterations were shared with Ewing sarcoma or desmoplastic, small round cell tumors, which are other EWSR1-rearranged tumors. Exome sequencing in mouse tumors generated by expression of EWSR1-ATF1 from the Rosa26 locus demonstrated no other repeated pathogenic variants. Additionally, we generated a new CCS mouse by Cre-loxP-induced chromosomal translocation between Ewsr1 and Atf1, resulting in copy number loss of chromosome 6 and chromosome 15 instability, including amplification of a portion syntenic to human chromosome 8, surrounding Myc. Additional experiments in the Rosa26 conditional model demonstrated that Mitf or Myc can contribute to sarcomagenesis. Copy number observations in human tumors and genetic experiments in mice rendered, for the first time to our knowledge, a functional landscape of the CCS genome. These data advance efforts to understand the biology of CCS using innovative models that will eventually allow us to validate preclinical therapies necessary to achieve longer and better survival for young patients with this disease.
Keywords: Cancer; Genetics; Mouse models; Oncogenes; Oncology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

