Extracellular matrix protein laminin β1 regulates pain sensitivity and anxiodepression-like behaviors in mice
- PMID: 34156983
- PMCID: PMC8321574
- DOI: 10.1172/JCI146323
Extracellular matrix protein laminin β1 regulates pain sensitivity and anxiodepression-like behaviors in mice
Abstract
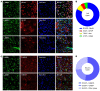
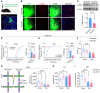
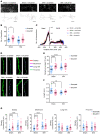
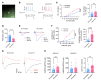
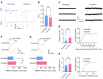
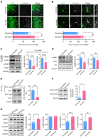
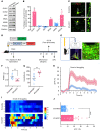
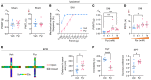
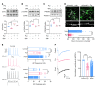
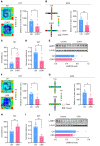
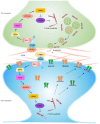
Patients with neuropathic pain often experience comorbid psychiatric disorders. Cellular plasticity in the anterior cingulate cortex (ACC) is assumed to be a critical interface for pain perception and emotion. However, substantial efforts have thus far been focused on the intracellular mechanisms of plasticity rather than the extracellular alterations that might trigger and facilitate intracellular changes. Laminin, a key element of the extracellular matrix (ECM), consists of one α-, one β-, and one γ-chain and is implicated in several pathophysiological processes. Here, we showed in mice that laminin β1 (LAMB1) in the ACC was significantly downregulated upon peripheral neuropathy. Knockdown of LAMB1 in the ACC exacerbated pain sensitivity and induced anxiety and depression. Mechanistic analysis revealed that loss of LAMB1 caused actin dysregulation via interaction with integrin β1 and the subsequent Src-dependent RhoA/LIMK/cofilin pathway, leading to increased presynaptic transmitter release probability and abnormal postsynaptic spine remodeling, which in turn orchestrated the structural and functional plasticity of pyramidal neurons and eventually resulted in pain hypersensitivity and anxiodepression. This study sheds new light on the functional capability of ECM LAMB1 in modulating pain plasticity and identifies a mechanism that conveys extracellular alterations to intracellular plasticity. Moreover, we identified cingulate LAMB1/integrin β1 signaling as a promising therapeutic target for the treatment of neuropathic pain and associated anxiodepression.
Keywords: Depression; Laminin; Neuroscience; Pain.
Conflict of interest statement
Figures

References
-
- McWilliams LA, et al. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111(1-2):77–83. - PubMed

