Field validation of a magneto-optical detection device (Gazelle) for portable point-of-care Plasmodium vivax diagnosis
- PMID: 34157032
- PMCID: PMC8219132
- DOI: 10.1371/journal.pone.0253232
Field validation of a magneto-optical detection device (Gazelle) for portable point-of-care Plasmodium vivax diagnosis
Abstract
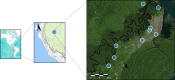
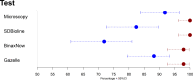
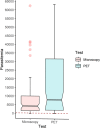
A major challenge for malaria is the lack of tools for accurate and timely diagnosis in the field which are critical for case management and surveillance. Microscopy along with rapid diagnostic tests are the current mainstay for malaria diagnosis in most endemic regions. However, these methods present several limitations. This study assessed the accuracy of Gazelle, a novel rapid malaria diagnostic device, from samples collected from the Peruvian Amazon between 2019 and 2020. Diagnostic accuracy was compared against microscopy and two rapid diagnostic tests (SD Bioline and BinaxNOW) using 18ssr nested-PCR as reference test. In addition, a real-time PCR assay (PET-PCR) was used for parasite quantification. Out of 217 febrile patients enrolled and tested, 180 specimens (85 P. vivax and 95 negatives) were included in the final analysis. Using nested-PCR as the gold standard, the sensitivity and specificity of Gazelle was 88.2% and 97.9%, respectively. Using a cutoff of 200 parasites/μl, Gazelle's sensitivity for samples with more than 200 p/uL was 98.67% (95%CI: 92.79% to 99.97%) whereas the sensitivity for samples lower than 200 p/uL (n = 10) was 12.5% (95%CI: 0.32% to 52.65%). Gazelle's sensitivity and specificity were statistically similar to microscopy (sensitivity = 91.8, specificity = 100%, p = 0.983) and higher than both SD Bioline (sensitivity = 82.4, specificity = 100%, p = 0.016) and BinaxNOW (sensitivity = 71.8%, specificity = 97.9%, p = 0.002). The diagnostic accuracy of Gazelle for malaria detection in P. vivax infections was comparable to light microscopy and superior to both RDTs even in the presence of low parasitemia infections. The performance of Gazelle makes it a valuable tool for malaria diagnosis and active case detection that can be utilized in different malaria-endemic regions.
Conflict of interest statement
PT is an employee of Hemex Health and reports personal fees and other from Hemex Health, KB is an employee of Vysnova and reports personal fees outside the submitted work. CAJ, DKB, HOV, GB, LR, KB, CS and DKB reports grants and non-financial support from Hemex Health, US during the conduct of the study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- WHO. World malaria report World Health Organization. 2019.
-
- Livezey J, Twomey P, Morrison M, Cicatelli S, Duncan EH, Hamer M, et al.. An open label study of the safety and efficacy of a single dose of weekly chloroquine and azithromycin administered for malaria prophylaxis in healthy adults challenged with 7G8 chloroquine-resistant Plasmodium falciparum in a controlled human malaria infection model. Malaria journal. 2020;19(1):336. doi: 10.1186/s12936-020-03409-z ; PubMed Central PMCID: PMC7493140. - DOI - PMC - PubMed
-
- Lo E, Zhou G, Oo W, Afrane Y, Githeko A, Yan G. Low parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of Western Kenya. PloS one. 2015;10(3):e0121763. doi: 10.1371/journal.pone.0121763 ; PubMed Central PMCID: PMC4376713. - DOI - PMC - PubMed
-
- Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, et al.. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PloS one. 2010;5(1):e8091. doi: 10.1371/journal.pone.0008091 ; PubMed Central PMCID: PMC2810332. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

