Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up
- PMID: 34157132
- PMCID: PMC9290992
- DOI: 10.1111/bjd.20595
Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up
Abstract
Background: Psoriasis is a chronic inflammatory skin disease requiring prolonged treatment. New biologic therapies require long-term evaluation to assess the durability of their efficacy and safety profiles over time.
Objectives: To evaluate the long-term efficacy and safety of risankizumab (RZB) for the treatment of psoriasis.
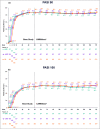
Methods: LIMMitless is an ongoing, phase III, open-label extension study evaluating the long-term efficacy and safety of RZB in adults with moderate-to-severe plaque psoriasis following multiple phase II/III studies. This analysis assessed efficacy through 172 weeks of continuous RZB treatment by examining the proportion of patients achieving ≥ 90% or 100% improvement in Psoriasis Area and Severity Index (PASI 90 and PASI 100), static Physician's Global Assessment of clear or almost clear (sPGA 0/1) and Dermatology Life Quality Index of no effect on quality of life (DLQI 0/1). Safety was assessed by recording adverse events (AEs) through the data cutoff date. The study is registered at ClinicalTrials.gov (identifier: NCT03047395).
Results: Of 955 patients randomized to RZB 150 mg in the base studies, 897 patients continued into LIMMitless; 799 patients were still receiving treatment in LIMMitless at the time of data cutoff for this analysis. After 172 weeks of continuous RZB treatment, 85·5% of patients achieved PASI 90, 54·4% achieved PASI 100, 85·2% achieved sPGA 0/1, and 78·4% achieved DLQI 0/1 using modified nonresponder imputation. Rates of AEs leading to discontinuation and AEs of safety interest were low with long-term treatment and comparable with those identified in the base studies.
Conclusions: Overall, long-term continuous RZB was well tolerated and showed high and durable efficacy over 172 weeks.
© 2021 AbbVie Inc. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures





Comment in
-
Long-term efficacy: the new gold standard?Br J Dermatol. 2021 Dec;185(6):1086-1087. doi: 10.1111/bjd.20715. Epub 2021 Oct 10. Br J Dermatol. 2021. PMID: 34632571 No abstract available.
Similar articles
-
Long-Term Safety and Efficacy of Risankizumab to Treat Moderate-to-Severe Plaque Psoriasis: Final LIMMitless Phase 3, Open-Label Extension Trial Results.Am J Clin Dermatol. 2025 Jul 29. doi: 10.1007/s40257-025-00964-6. Online ahead of print. Am J Clin Dermatol. 2025. PMID: 40728772
-
Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: Interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up.J Am Acad Dermatol. 2023 Dec;89(6):1149-1158. doi: 10.1016/j.jaad.2023.07.1024. Epub 2023 Aug 6. J Am Acad Dermatol. 2023. PMID: 37553030 Clinical Trial.
-
Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients With Moderate to Severe Plaque Psoriasis: A Phase 3 Randomized Clinical Trial.JAMA Dermatol. 2020 Jun 1;156(6):649-658. doi: 10.1001/jamadermatol.2020.0723. JAMA Dermatol. 2020. PMID: 32267471 Free PMC article. Clinical Trial.
-
Risankizumab: A Review in Moderate to Severe Plaque Psoriasis.Drugs. 2020 Aug;80(12):1235-1245. doi: 10.1007/s40265-020-01357-1. Drugs. 2020. PMID: 32632826 Free PMC article. Review.
-
The efficacy and safety of tyrosine kinase 2 inhibitor deucravacitinib in the treatment of plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials.Front Med (Lausanne). 2023 Sep 29;10:1264667. doi: 10.3389/fmed.2023.1264667. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37841017 Free PMC article.
Cited by
-
Positioning risankizumab in the treatment algorithm of moderate-to-severe Crohn's disease.Immunotherapy. 2024;16(9):581-595. doi: 10.2217/imt-2023-0219. Epub 2024 Apr 17. Immunotherapy. 2024. PMID: 38629330 Free PMC article. Review.
-
Risk of Melanoma and Non-Melanoma Skin Cancer in Patients with Psoriasis and Psoriatic Arthritis Treated with Targeted Therapies: A Systematic Review and Meta-Analysis.Pharmaceuticals (Basel). 2023 Dec 21;17(1):14. doi: 10.3390/ph17010014. Pharmaceuticals (Basel). 2023. PMID: 38276003 Free PMC article. Review.
-
Risankizumab for the Treatment of Moderate to Severe Psoriasis: Impact on Health-Related Quality of Life and Psychological Wellbeing.Clin Cosmet Investig Dermatol. 2023 Jan 25;16:221-229. doi: 10.2147/CCID.S296544. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 36721838 Free PMC article. Review.
-
Safety of IL-23 p19 Inhibitors for the Treatment of Patients With Moderate-to-Severe Plaque Psoriasis: A Narrative Review.Adv Ther. 2023 Aug;40(8):3410-3433. doi: 10.1007/s12325-023-02568-0. Epub 2023 Jun 18. Adv Ther. 2023. PMID: 37330926 Free PMC article. Review.
-
Interleukin inhibitors and the associated risk of candidiasis.Front Immunol. 2024 Mar 28;15:1372693. doi: 10.3389/fimmu.2024.1372693. eCollection 2024. Front Immunol. 2024. PMID: 38605952 Free PMC article. Review.
References
-
- Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol 2017; 13:525–34. - PubMed
-
- Skyrizi (risankizumab‐rzaa) [package insert]. Chicago, IL: AbbVie Inc., 2020.

