Immunomodulatory Effects of Lenvatinib Plus Anti-Programmed Cell Death Protein 1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma
- PMID: 34157147
- PMCID: PMC12155127
- DOI: 10.1002/hep.32023
Immunomodulatory Effects of Lenvatinib Plus Anti-Programmed Cell Death Protein 1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma
Abstract
Background and aims: Lenvatinib is an effective drug in advanced HCC. Its combination with the anti-PD1 (programmed cell death protein 1) immune checkpoint inhibitor, pembrolizumab, has generated encouraging results in phase Ib and is currently being tested in phase III trials. Here, we aimed to explore the molecular and immunomodulatory effects of lenvatinib alone or in combination with anti-PD1.
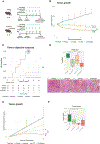
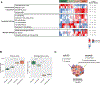
Approach and results: We generated three syngeneic models of HCC in C57BL/6J mice (subcutaneous and orthotopic) and randomized animals to receive placebo, lenvatinib, anti-PD1, or combination treatment. Flow cytometry, transcriptomic, and immunohistochemistry analyses were performed in tumor and blood samples. A gene signature, capturing molecular features associated with the combination therapy, was used to identify a subset of candidates in a cohort of 228 HCC patients who might respond beyond what is expected for monotherapies. In mice, the combination treatment resulted in tumor regression and shorter time to response compared to monotherapies (P < 0.001). Single-agent anti-PD1 induced dendritic and T-cell infiltrates, and lenvatinib reduced the regulatory T cell (Treg) proportion. However, only the combination treatment significantly inhibited immune suppressive signaling, which was associated with the TGFß pathway and induced an immune-active microenvironment (P < 0.05 vs. other therapies). Based on immune-related genomic profiles in human HCC, 22% of patients were identified as potential responders beyond single-agent therapies, with tumors characterized by Treg cell infiltrates, low inflammatory signaling, and VEGFR pathway activation.
Conclusions: Lenvatinib plus anti-PD1 exerted unique immunomodulatory effects through activation of immune pathways, reduction of Treg cell infiltrate, and inhibition of TGFß signaling. A gene signature enabled the identification of ~20% of human HCCs that, although nonresponding to single agents, could benefit from the proposed combination.
© 2021 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Dr. Leslie owns stock in Fibrofind. Dr. Llovet consults for and received grants from Bayer, Eisai, Boehringer Ingelheim, Bristol-Myers Squibb, and Ipsen. He consults for Celsion, Eli Lilly, Merck, Roche, Genentech, Glycotest, Nucleix, Can-Fite, Sirtex, AstraZeneca, and Mina Alpha. Dr. Friedman consults for, received grants from, and owns stock in Morphic Therapeutics and Galmed. He consults for and owns stock in Blade, Escient, Glympse, North Sea, Scholar Rock, and Surrozen. He consults for 89 Bio, Amgen, Axcella, Bristol-Myers Squibb, Can-Fite, ChemomAb, Forbion, Gordion, Glycotest, In sitro, Novartis, Ono, and Pfizer. He received grants from Novo Nordisk and Abalone. He owns stock in Galectin, Genfit, Lifemax, Metacrine, Nimbus, Intercept, Madrigal, and Group K. Dr. Mann is employed by and owns stock in Fibrofind. He received grants from GlaxoSmithKline.
Figures






References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. - PubMed
-
- Cheng AL, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 2017;35(Suppl.):Abstract 4001.
-
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–1905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

