Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination
- PMID: 34157170
- PMCID: PMC8373720
- DOI: 10.1002/glia.24049
Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination
Abstract
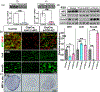
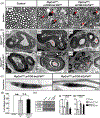
Multiple signals are involved in the regulation of developmental myelination by Schwann cells and in the maintenance of a normal myelin homeostasis throughout adult life, preserving the integrity of the axons in the PNS. Recent studies suggest that Mek/ERK1/2-MAPK and PI3K/Akt/mTOR intracellular signaling pathways play important, often overlapping roles in the regulation of myelination in the PNS. In addition, hyperactivation of these signaling pathways in Schwann cells leads to a late onset of various pathological changes in the sciatic nerves. However, it remains poorly understood whether these pathways function independently or sequentially or converge using a common mechanism to facilitate Schwann cell differentiation and myelin growth during development and in causing pathological changes in the adult animals. To address these questions, we analyzed multiple genetically modified mice using simultaneous loss- and constitutive gain-of-function approaches. We found that during development, the Mek/ERK1/2-MAPK pathway plays a primary role in Schwann cell differentiation, distinct from mTOR. However, during active myelination, ERK1/2 is dependent on mTOR signaling to drive the growth of the myelin sheath and regulate its thickness. Finally, our data suggest that peripheral nerve pathology during adulthood caused by hyperactivation of Mek/ERK1/2-MAPK or PI3K is likely to be independent or dependent on mTOR-signaling in different contexts. Thus, this study highlights the complexities in the roles played by two major intracellular signaling pathways in Schwann cells that affect their differentiation, myelination, and later PNS pathology and predicts that potential therapeutic modulation of these pathways in PNS neuropathies could be a complex process.
Keywords: Schwann cells; differentiation; myelin; myelination.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Figures






Similar articles
-
Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood.Glia. 2019 Jul;67(7):1277-1295. doi: 10.1002/glia.23602. Epub 2019 Feb 13. Glia. 2019. PMID: 30761608 Free PMC article.
-
Akt Regulates Axon Wrapping and Myelin Sheath Thickness in the PNS.J Neurosci. 2016 Apr 20;36(16):4506-21. doi: 10.1523/JNEUROSCI.3521-15.2016. J Neurosci. 2016. PMID: 27098694 Free PMC article.
-
Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS.J Neurosci. 2016 Jun 15;36(24):6471-87. doi: 10.1523/JNEUROSCI.0299-16.2016. J Neurosci. 2016. PMID: 27307235 Free PMC article.
-
Akt/mTOR signalling in myelination.Biochem Soc Trans. 2013 Aug;41(4):944-50. doi: 10.1042/BST20130046. Biochem Soc Trans. 2013. PMID: 23863161 Review.
-
Myelination and mTOR.Glia. 2018 Apr;66(4):693-707. doi: 10.1002/glia.23273. Epub 2017 Dec 6. Glia. 2018. PMID: 29210103 Free PMC article. Review.
Cited by
-
Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications.Biomedicines. 2024 Feb 22;12(3):489. doi: 10.3390/biomedicines12030489. Biomedicines. 2024. PMID: 38540102 Free PMC article. Review.
-
Mechanosensitive Piezo1 channel: an emerging target in demyelination disease.Front Cell Neurosci. 2025 Jul 9;19:1556892. doi: 10.3389/fncel.2025.1556892. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40703567 Free PMC article. Review.
-
Signaling Pathways Controlling Axonal Wrapping in Drosophila.Cells. 2023 Oct 31;12(21):2553. doi: 10.3390/cells12212553. Cells. 2023. PMID: 37947631 Free PMC article. Review.
-
The factors affecting neurogenesis after stroke and the role of acupuncture.Front Neurol. 2023 Jan 20;14:1082625. doi: 10.3389/fneur.2023.1082625. eCollection 2023. Front Neurol. 2023. PMID: 36741282 Free PMC article. Review.
-
Roles of ERK signaling pathway in regulating myelination of the peripheral nervous system.Front Mol Neurosci. 2025 Jun 13;18:1617976. doi: 10.3389/fnmol.2025.1617976. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40584403 Free PMC article. Review.
References
-
- Beirowski B, Wong KM, Babetto E, & Milbrandt J. (2017). mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells. Proceedings of the National Academy of Sciences of the United States of America, 114, E4261–E4270. 10.1073/pnas.1620761114 - DOI - PMC - PubMed
-
- Cotter L, Ozçelik M, Jacob C, Pereira JA, Locher V, Baumann R, Relvas JB, Suter U, & Tricaud N. (2010). Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve over-myelination. Science, 328, 1415–1418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

