Co-immunization with L-Myc enhances CD8+ or CD103+ DCs mediated tumor-specific multi-functional CD8+ T cell responses
- PMID: 34157192
- PMCID: PMC8409417
- DOI: 10.1111/cas.15044
Co-immunization with L-Myc enhances CD8+ or CD103+ DCs mediated tumor-specific multi-functional CD8+ T cell responses
Abstract
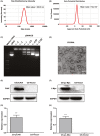
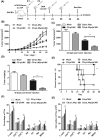
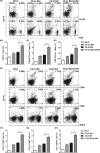
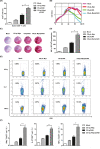
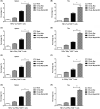
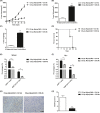
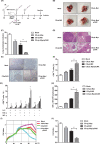
Renal carcinoma shows a high risk of invasion and metastasis without effective treatment. Herein, we developed a chitosan (CS) nanoparticle-mediated DNA vaccine containing an activated factor L-Myc and a tumor-specific antigen CAIX for renal carcinoma treatment. The subcutaneous tumor models were intramuscularly immunized with CS-pL-Myc/pCAIX or control vaccine, respectively. Compared with single immunization group, the tumor growth was significantly suppressed in CS-pL-Myc/pCAIX co-immunization group. The increased proportion and mature of CD11c+ DCs, CD8+ CD11c+ DCs and CD103+ CD11c+ DCs were observed in the splenocytes from CS-pL-Myc/pCAIX co-immunized mice. Furthermore, the enhanced antigen-specific CD8+ T lymphocyte proliferation, cytotoxic T lymphocyte (CTL) responses, and multi-functional CD8+ T cell induction were detected in CS-pL-Myc/pCAIX co-immunization group compared with CS-pCAIX immunization group. Of note, the depletion of CD8 T cells resulted in the reduction of CD8+ T cells or CD8+ CD11c+ DCs and the loss of anti-tumor efficacy induced by CS-pL-Myc/pCAIX vaccine, suggesting the therapeutic efficacy of the vaccine was required for CD8+ DCs and CD103+ DCs mediated CD8+ T cells responses. Likewise, CS-pL-Myc/pCAIX co-immunization also significantly inhibited the lung metastasis of renal carcinoma models accompanied with the increased induction of multi-functional CD8+ T cell responses. Therefore, these results indicated that CS-pL-Myc/pCAIX vaccine could effectively induce CD8+ DCs and CD103+ DCs mediated tumor-specific multi-functional CD8+ T cell responses and exert the anti-tumor efficacy. This vaccine strategy offers a potential and promising approach for solid or metastatic tumor treatment.
Keywords: CAIX; CS nanoparticles; L-Myc; multi-functional CD8+ T cells; renal carcinoma; tumor vaccine.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Combining DNA Vaccine and AIM2 in H1 Nanoparticles Exert Anti-Renal Carcinoma Effects via Enhancing Tumor-Specific Multi-functional CD8+ T-cell Responses.Mol Cancer Ther. 2019 Feb;18(2):323-334. doi: 10.1158/1535-7163.MCT-18-0832. Epub 2018 Nov 6. Mol Cancer Ther. 2019. PMID: 30401695
-
Adenovirus vaccine therapy with CD137L promotes CD8+ DCs-mediated multifunctional CD8+ T cell immunity and elicits potent anti-tumor activity.Pharmacol Res. 2022 Jan;175:106034. doi: 10.1016/j.phrs.2021.106034. Epub 2021 Dec 14. Pharmacol Res. 2022. PMID: 34915126
-
Co-immunization with IFI35 enhances the therapeutic effect of an adenovirus vaccine against renal carcinoma.Int J Biol Macromol. 2025 Jan;286:138515. doi: 10.1016/j.ijbiomac.2024.138515. Epub 2024 Dec 6. Int J Biol Macromol. 2025. PMID: 39647736
-
Hyper-interleukin-11 novel designer molecular adjuvant targeting gp130 for whole cell cancer vaccines.Expert Opin Biol Ther. 2011 Dec;11(12):1555-67. doi: 10.1517/14712598.2011.627852. Epub 2011 Oct 14. Expert Opin Biol Ther. 2011. PMID: 21995459 Review.
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
Cited by
-
The co-delivery of adenovirus-based immune checkpoint vaccine elicits a potent anti-tumor effect in renal carcinoma.NPJ Vaccines. 2023 Aug 4;8(1):109. doi: 10.1038/s41541-023-00706-x. NPJ Vaccines. 2023. PMID: 37542081 Free PMC article.
-
Nanomedicine for renal cell carcinoma: imaging, treatment and beyond.J Nanobiotechnology. 2023 Jan 3;21(1):3. doi: 10.1186/s12951-022-01761-7. J Nanobiotechnology. 2023. PMID: 36597108 Free PMC article. Review.
-
Effective strategies to enhance the diagnosis and treatment of RCC: The application of biocompatible materials.Mater Today Bio. 2024 Jul 9;27:101149. doi: 10.1016/j.mtbio.2024.101149. eCollection 2024 Aug. Mater Today Bio. 2024. PMID: 39100279 Free PMC article. Review.
-
Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy.Cancers (Basel). 2022 May 17;14(10):2473. doi: 10.3390/cancers14102473. Cancers (Basel). 2022. PMID: 35626078 Free PMC article. Review.
-
Dual-targeting vaccine of FGL1/CAIX exhibits potent anti-tumor activity by activating DC-mediated multi-functional CD8 T cell immunity.Mol Ther Oncolytics. 2021 Nov 29;24:1-13. doi: 10.1016/j.omto.2021.11.017. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2021. PMID: 34977338 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- TD202003/Youth Technology Innovation Team of Xuzhou Medical University
- 82072814/National Natural Science Foundation of China
- 81871869/National Natural Science Foundation of China
- BK20190986/Natural Science Foundation of Jiangsu Province
- KC19082/Key Research Development project of Xuzhou (Industry Foresight and Common Key Technology)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

