Application of a dual mechanistic approach to support bilastine dose selection for older adults
- PMID: 34157202
- PMCID: PMC8452293
- DOI: 10.1002/psp4.12671
Application of a dual mechanistic approach to support bilastine dose selection for older adults
Abstract
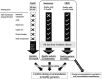
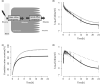
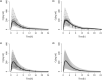
The objective of this study was to evaluate bilastine dosing recommendations in older adults and overcome the limitation of insufficient data from phase I studies in this underrepresented population. This was achieved by integrating bilastine physicochemical, in vitro and in vivo data in young adults and the effect of aging in the pharmacology by means of two alternative approaches: a physiologically-based pharmacokinetic (PBPK) model and a semi-mechanistic population pharmacokinetic (Senescence) model. Intestinal apical efflux and basolateral influx transporters were needed in the PBPK model to capture the observations from young adults after single i.v. (10 mg) and p.o. (20 mg) doses, supporting the hypothesis of involvement of gut transporters on secretion. The model was then used to extrapolate the pharmacokinetics (PKs) to elderly subjects considering their specific physiology. Additionally, the Senescence model was develop starting from a published population PK) model, previously applied for pediatrics, and incorporating declining functions on different physiological systems and changes in body composition with aging. Both models were qualified using observed data in a small group of young elderlies (N = 16, mean age = 68.69 years). The PBPK model was further used to evaluate the dose in older subjects (mean age = 80 years) via simulation. The PBPK model supported the hypothesis that basolateral influx and apical efflux transporters are involved in bilastine PK. Both, PBPK and Senescence models indicated that a 20 mg q.d. dose is safe and effective for geriatrics of any age. This approach provides an alternative to generate supplementary data to inform dosing recommendations in under-represented groups in clinical trials.
© 2021 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
C.C. and A.G.‐B. are employees of FAES FARMA, S.A. All other authors declared no competing interests for this work.
Figures
Similar articles
-
Model Informed Pediatric Development Applied to Bilastine: Ontogenic PK Model Development, Dose Selection for First Time in Children and PK Study Design.Pharm Res. 2017 Dec;34(12):2720-2734. doi: 10.1007/s11095-017-2248-6. Epub 2017 Oct 2. Pharm Res. 2017. PMID: 28971281 Clinical Trial.
-
Model-informed pediatric development applied to bilastine: Analysis of the clinical PK data and confirmation of the dose selected for the target population.Eur J Pharm Sci. 2019 Feb 1;128:180-192. doi: 10.1016/j.ejps.2018.11.016. Epub 2018 Nov 22. Eur J Pharm Sci. 2019. PMID: 30468868
-
Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastine.Clin Pharmacokinet. 2009;48(8):543-54. doi: 10.2165/11317180-000000000-00000. Clin Pharmacokinet. 2009. PMID: 19705924 Clinical Trial.
-
Bilastine: a new H1 -antihistamine with an optimal profile for updosing in urticaria.J Eur Acad Dermatol Venereol. 2017 Sep;31(9):1447-1452. doi: 10.1111/jdv.14305. Epub 2017 Jun 1. J Eur Acad Dermatol Venereol. 2017. PMID: 28467671 Review.
-
Role of Bilastine in Allergic Rhinitis: A Narrative Review.J Assoc Physicians India. 2023 Nov;71(11):58-61. doi: 10.59556/japi.71.0401. J Assoc Physicians India. 2023. PMID: 38720498 Review.
Cited by
-
From In Vivo Predictive Dissolution to Virtual Bioequivalence: A GastroPlus®-Driven Framework for Generic Candesartan Cilexetil Tablets.Pharmaceuticals (Basel). 2025 Apr 11;18(4):562. doi: 10.3390/ph18040562. Pharmaceuticals (Basel). 2025. PMID: 40283997 Free PMC article.
-
Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability.Antibiotics (Basel). 2022 May 11;11(5):641. doi: 10.3390/antibiotics11050641. Antibiotics (Basel). 2022. PMID: 35625285 Free PMC article. Review.
-
Apixaban and rivaroxaban's physiologically-based pharmacokinetic model validation in hospitalized patients: A first step for larger use of a priori modeling approach at bed side.CPT Pharmacometrics Syst Pharmacol. 2023 Dec;12(12):1872-1883. doi: 10.1002/psp4.13036. Epub 2023 Oct 4. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 37794718 Free PMC article.
References
-
- Corcostegui R, Labeaga L, Innerarity A, Berisa A, Orjales A. In vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonist. Drugs R & D. 2006;7(4):219‐231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

