A local regulatory T cell feedback circuit maintains immune homeostasis by pruning self-activated T cells
- PMID: 34157301
- PMCID: PMC8390950
- DOI: 10.1016/j.cell.2021.05.028
A local regulatory T cell feedback circuit maintains immune homeostasis by pruning self-activated T cells
Abstract
A fraction of mature T cells can be activated by peripheral self-antigens, potentially eliciting host autoimmunity. We investigated homeostatic control of self-activated T cells within unperturbed tissue environments by combining high-resolution multiplexed and volumetric imaging with computational modeling. In lymph nodes, self-activated T cells produced interleukin (IL)-2, which enhanced local regulatory T cell (Treg) proliferation and inhibitory functionality. The resulting micro-domains reciprocally constrained inputs required for damaging effector responses, including CD28 co-stimulation and IL-2 signaling, constituting a negative feedback circuit. Due to these local constraints, self-activated T cells underwent transient clonal expansion, followed by rapid death ("pruning"). Computational simulations and experimental manipulations revealed the feedback machinery's quantitative limits: modest reductions in Treg micro-domain density or functionality produced non-linear breakdowns in control, enabling self-activated T cells to subvert pruning. This fine-tuned, paracrine feedback process not only enforces immune homeostasis but also establishes a sharp boundary between autoimmune and host-protective T cell responses.
Keywords: CTLA-4; IL-2; IL-2Rα; apoptosis; autoimmunity; computational modeling; feedback control; immune homeostasis; quantitative tissue imaging; regulatory T cells.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests A.Y.R. is a co-founder of Sonoma Biotherapeutics; he is an SAB member and reports personal fees from Sonoma Biotherapeutics, RAPT Therapeutics, and Vedanta Biosciences and holds an IP licensed to Takeda all outside the submitted work. All other authors declare no competing interests.
Figures
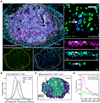


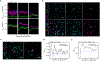



Comment in
-
Volume control: Turning the dial on regulatory T cells.Cell. 2021 Jul 22;184(15):3847-3849. doi: 10.1016/j.cell.2021.06.015. Cell. 2021. PMID: 34297928
References
-
- Akbar AN, Borthwick N, Salmon M, Gombert W, Bofill M, Shamsadeen N, Pilling D, Pett S, Grundy JE, and Janossy G (1993). The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J. Exp. Med 178, 427–438. - PMC - PubMed
-
- Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, and Salmon M (1996). Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol 26, 294–299. - PubMed
-
- Almeida ARM, Zaragoza B, and Freitas AA (2006). Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J. Immunol 177, 192–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

