Green Light Antinociceptive and Reversal of Thermal and Mechanical Hypersensitivity Effects Rely on Endogenous Opioid System Stimulation
- PMID: 34157406
- PMCID: PMC8664962
- DOI: 10.1016/j.jpain.2021.05.006
Green Light Antinociceptive and Reversal of Thermal and Mechanical Hypersensitivity Effects Rely on Endogenous Opioid System Stimulation
Abstract
Benefits of phototherapy were characterized in multiple diseases including depression, circadian rhythm disruptions, and neurodegeneration. Studies on migraine and fibromyalgia patients revealed that green light-emitting diodes (GLED) exposure provides a pragmatic and safe therapy to manage chronic pain. In rodents, GLED reversed hypersensitivity related to neuropathic pain. However, little is known about the underlying mechanisms of GLED efficacy. Here, we sought to understand how green light modulates the endogenous opioid system. We first characterized how exposure to GLED stimulates release of β-endorphin and proenkephalin in the central nervous system of male rats. Moreover, by individually editing each of the receptors, we found that µ- and δ-opioid receptors are required for green light's antinociceptive effect in naïve rats and a model of HIV-induced peripheral neuropathy. We investigated how GLED could increase pain thresholds, and explored its potential in reversing hypersensitivity in a model of HIV-related neuropathy. Through behavioral and gene editing approaches, we identified that green light provides antinociception via modulation of the endogenous opioid system in the spinal cord. This work identifies a previously unknown mechanism by which GLED can improve pain management. Clinical translation of these results will advance the development of an innovative therapy devoid of adverse effects. PERSPECTIVE: Development of new pain management therapies, especially for HIV patients, is crucial as long-term opioid prescription is not recommended due to adverse side effects. Green light addresses this necessity. Characterizing the underlying mechanisms of this potentially groundbreaking and safe antinociceptive therapy will advance its clinical translation.
Keywords: GP120; Phototherapy; endogenous opioids; green light; neuropathic pain.
Copyright © 2021 United States Association for the Study of Pain, Inc. Published by Elsevier Inc. All rights reserved.
Figures


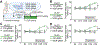
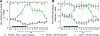
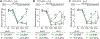
Similar articles
-
Green Light Exposure Elicits Anti-inflammation, Endogenous Opioid Release and Dampens Synaptic Potentiation to Relieve Post-surgical Pain.J Pain. 2023 Mar;24(3):509-529. doi: 10.1016/j.jpain.2022.10.011. Epub 2022 Oct 23. J Pain. 2023. PMID: 36283655 Free PMC article.
-
Long-lasting antinociceptive effects of green light in acute and chronic pain in rats.Pain. 2017 Feb;158(2):347-360. doi: 10.1097/j.pain.0000000000000767. Pain. 2017. PMID: 28092651 Free PMC article.
-
Low frequency electroacupuncture alleviates neuropathic pain by activation of spinal microglial IL-10/β-endorphin pathway.Biomed Pharmacother. 2020 May;125:109898. doi: 10.1016/j.biopha.2020.109898. Epub 2020 Jan 29. Biomed Pharmacother. 2020. PMID: 32004977
-
Alterations in the Activity of Spinal and Thalamic Opioid Systems in a Mice Neuropathic Pain Model.Neuroscience. 2018 Oct 15;390:293-302. doi: 10.1016/j.neuroscience.2018.08.013. Epub 2018 Sep 1. Neuroscience. 2018. PMID: 30176322
-
Opioids in chronic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):79-91. doi: 10.1016/s0014-2999(01)01308-5. Eur J Pharmacol. 2001. PMID: 11698029 Review.
Cited by
-
A male-specific mechanism of meningeal nociceptor sensitization promoting migraine headache.Cephalalgia. 2024 Sep;44(9):3331024241281493. doi: 10.1177/03331024241281493. Cephalalgia. 2024. PMID: 39233656 Free PMC article.
-
Narrow band green light effects on headache, photophobia, sleep, and anxiety among migraine patients: an open-label study conducted online using daily headache diary.Front Neurol. 2023 Oct 4;14:1282236. doi: 10.3389/fneur.2023.1282236. eCollection 2023. Front Neurol. 2023. PMID: 37859647 Free PMC article.
-
A Comprehensive Overview of the Neural Mechanisms of Light Therapy.Neurosci Bull. 2024 Mar;40(3):350-362. doi: 10.1007/s12264-023-01089-8. Epub 2023 Aug 9. Neurosci Bull. 2024. PMID: 37555919 Free PMC article. Review.
-
Evaluating the Potential of Green Light Exposure on Nociception-A Mini Review.CNS Neurol Disord Drug Targets. 2024;23(6):675-679. doi: 10.2174/1871527322666230522105931. CNS Neurol Disord Drug Targets. 2024. PMID: 37221686 Review.
-
The conotoxin Contulakin-G reverses hypersensitivity observed in rodent models of cancer-induced bone pain without inducing tolerance or motor disturbance.Pain. 2025 Feb 1;166(2):376-387. doi: 10.1097/j.pain.0000000000003391. Epub 2024 Sep 19. Pain. 2025. PMID: 39297754
References
-
- Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O’Donnell D, Kieffer BL, Woodbury CJ, Basbaum AI, MacDermott AB, Scherrer G. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 81:1312–1327, 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

