Three diketomorpholines from a Penicillium sp. (strain G1071)
- PMID: 34157637
- PMCID: PMC8292221
- DOI: 10.1016/j.phytochem.2021.112830
Three diketomorpholines from a Penicillium sp. (strain G1071)
Abstract
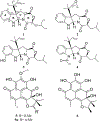
Three previously undescribed diketomorpholine natural products, along with the known phenalenones, herqueinone and norherqueinone, were isolated from the mycoparasitic fungal strain G1071, which was identified as a Penicillium sp. in the section Sclerotiora. The structures were established by analyzing NMR data and mass spectrometry fragmentation patterns. The absolute configurations of deacetyl-javanicunine A, javanicunine C, and javanicunine D, were assigned by examining ECD spectra and Marfey's analysis. The structural diversity generated by this fungal strain was interesting, as only a few diketomorpholines (~17) have been reported from nature.
Keywords: Diketomorpholine; Javanicunine; Penicillium; Phenalenones; Pyrrolidinoindoline.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
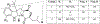
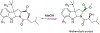
References
-
- Afiyatullov SS, Kalinovskii AI, Pivkin MV, Dmitrenok PS, Kuznetsova TA, 2004. Fumitremorgins from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd 40, 615–617. DOI: 10.1007/s10600-005-0058-2. - DOI
-
- Arai K, Kimura K, Mushiroda T, Yamamoto Y, 1989. Structures of fructigenines A and B, new alkaloids isolated from Penicillium fructigenum Takeuchi. Chem. Pharm. Bull 37, 2937–2939. DOI: 10.1248/cpb.37.2937. - DOI
-
- Ayers S, Ehrmann BM, Adcock AF, Kroll DJ, Carcache de Blanco EJ, Shen Q, Swanson SM, Falkinham JO 3rd, Wani MC, Mitchell SM, Pearce CJ, Oberlies NH, 2012. Peptaibols from two unidentified fungi of the order Hypocreales with cytotoxic, antibiotic, and anthelmintic activities. J. Pept. Sci 18, 500–510. DOI: 10.1002/psc.2425. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

