Protein arginine methyltransferase 1 regulates cell proliferation and differentiation in adult mouse adult intestine
- PMID: 34158114
- PMCID: PMC8220849
- DOI: 10.1186/s13578-021-00627-z
Protein arginine methyltransferase 1 regulates cell proliferation and differentiation in adult mouse adult intestine
Abstract
Background: Adult stem cells play an essential role in adult organ physiology and tissue repair and regeneration. While much has been learnt about the property and function of various adult stem cells, the mechanisms of their development remain poorly understood in mammals. Earlier studies suggest that the formation of adult mouse intestinal stem cells takes place during the first few weeks after birth, the postembryonic period when plasma thyroid hormone (T3) levels are high. Furthermore, deficiency in T3 signaling leads to defects in adult mouse intestine, including reduced cell proliferation in the intestinal crypts, where stem cells reside. Our earlier studies have shown that protein arginine methyltransferase 1 (PRMT1), a T3 receptor coactivator, is highly expressed during intestinal maturation in mouse.
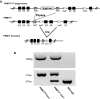
Methods: We have analyzed the expression of PRMT1 by immunohistochemistry and studied the effect of tissue-specific knockout of PRMT1 in the intestinal epithelium.
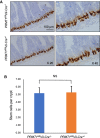
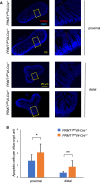
Results: We show that PRMT1 is expressed highly in the proliferating transit amplifying cells and crypt base stem cells. By using a conditional knockout mouse line, we have demonstrated that the expression of PRMT1 in the intestinal epithelium is critical for the development of the adult mouse intestine. Specific removal of PRMT1 in the intestinal epithelium results in, surprisingly, more elongated adult intestinal crypts with increased cell proliferation. In addition, epithelial cell migration along the crypt-villus axis and cell death on the villus are also increased. Furthermore, there are increased Goblet cells and reduced Paneth cells in the crypt while the number of crypt base stem cells remains unchanged.
Conclusions: Our finding that PRMT1 knockout increases cell proliferation is surprising considering the role of PRMT1 in T3-signaling and the importance of T3 for intestinal development, and suggests that PRMT1 likely regulates pathways in addition to T3-signaling to affect intestinal development and/or homeostasis, thus affecting cell proliferating and epithelial turn over in the adult.
Keywords: Adult organ-specific stem cell; Histone arginine methyltransferase; Intestine; Thyroid hormone receptor; Transcription coactivator.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Protein arginine methyltransferase 1 regulates mouse enteroendocrine cell development and homeostasis.Cell Biosci. 2024 Jun 4;14(1):70. doi: 10.1186/s13578-024-01257-x. Cell Biosci. 2024. PMID: 38835047 Free PMC article.
-
Thyroid hormone activates protein arginine methyltransferase 1 expression by directly inducing c-Myc transcription during Xenopus intestinal stem cell development.J Biol Chem. 2012 Mar 23;287(13):10039-10050. doi: 10.1074/jbc.M111.335661. Epub 2012 Feb 7. J Biol Chem. 2012. PMID: 22315222 Free PMC article.
-
Protein arginine methyltransferase 1 is required for the maintenance of adult small intestinal and colonic epithelial cell homeostasis.Int J Biol Sci. 2024 Jan 1;20(2):554-568. doi: 10.7150/ijbs.89958. eCollection 2024. Int J Biol Sci. 2024. PMID: 38169732 Free PMC article.
-
Thyroid hormone regulation of adult intestinal stem cell development: mechanisms and evolutionary conservations.Int J Biol Sci. 2012;8(8):1217-24. doi: 10.7150/ijbs.5109. Epub 2012 Oct 23. Int J Biol Sci. 2012. PMID: 23136549 Free PMC article. Review.
-
The balance of two opposing factors Mad and Myc regulates cell fate during tissue remodeling.Cell Biosci. 2018 Sep 15;8:51. doi: 10.1186/s13578-018-0249-8. eCollection 2018. Cell Biosci. 2018. PMID: 30237868 Free PMC article. Review.
Cited by
-
Overexpression of Circular PRMT1 Transcripts in Colorectal Adenocarcinoma Predicts Recurrence and Poor Overall Survival.Int J Mol Sci. 2025 Jul 11;26(14):6683. doi: 10.3390/ijms26146683. Int J Mol Sci. 2025. PMID: 40724932 Free PMC article.
-
PRMT1 in human neoplasm: cancer biology and potential therapeutic target.Cell Commun Signal. 2024 Feb 8;22(1):102. doi: 10.1186/s12964-024-01506-z. Cell Commun Signal. 2024. PMID: 38326807 Free PMC article. Review.
-
Effects of hormones on intestinal stem cells.Stem Cell Res Ther. 2023 Apr 26;14(1):105. doi: 10.1186/s13287-023-03336-1. Stem Cell Res Ther. 2023. PMID: 37101229 Free PMC article. Review.
-
The Potential Reversible Transition between Stem Cells and Transient-Amplifying Cells: The Limbal Epithelial Stem Cell Perspective.Cells. 2024 Apr 25;13(9):748. doi: 10.3390/cells13090748. Cells. 2024. PMID: 38727284 Free PMC article. Review.
-
Protein arginine methyltransferase 1 regulates mouse enteroendocrine cell development and homeostasis.Cell Biosci. 2024 Jun 4;14(1):70. doi: 10.1186/s13578-024-01257-x. Cell Biosci. 2024. PMID: 38835047 Free PMC article.
References
-
- Toner PG, Carr KE, Wyburn GM. The digestive system: an ultrastructural atlas and review. London: Butterworth; 1971.
Grants and funding
LinkOut - more resources
Full Text Sources

