5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAFV600 Mutation-Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study
- PMID: 34158360
- PMCID: PMC9401485
- DOI: 10.1158/1078-0432.CCR-21-0809
5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAFV600 Mutation-Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study
Abstract
Purpose: The randomized phase III coBRIM study (NCT01689519) demonstrated improved progression-free survival (PFS) and overall survival (OS) with addition of cobimetinib to vemurafenib compared with vemurafenib in patients with previously untreated BRAFV600 mutation-positive advanced melanoma. We report long-term follow-up of coBRIM, with at least 5 years since the last patient was randomized.
Patients and methods: Eligible patients were randomized 1:1 to receive either oral cobimetinib (60 mg once daily on days 1-21 in each 28-day cycle) or placebo in combination with oral vemurafenib (960 mg twice daily).
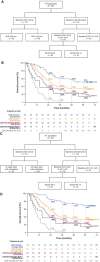
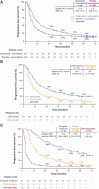
Results: 495 patients were randomized to cobimetinib plus vemurafenib (n = 247) or placebo plus vemurafenib (n = 248). Median follow-up was 21.2 months for cobimetinib plus vemurafenib and 16.6 months for placebo plus vemurafenib. Median OS was 22.5 months (95% CI, 20.3-28.8) with cobimetinib plus vemurafenib and 17.4 months (95% CI, 15.0-19.8) with placebo plus vemurafenib; 5-year OS rates were 31% and 26%, respectively. Median PFS was 12.6 months (95% CI, 9.5-14.8) with cobimetinib plus vemurafenib and 7.2 months (95% CI, 5.6-7.5) with placebo plus vemurafenib; 5-year PFS rates were 14% and 10%, respectively. OS and PFS were longest in patients with normal baseline lactate dehydrogenase levels and low tumor burden, and in those achieving complete response. The safety profile remained consistent with previously published reports.
Conclusions: Extended follow-up of coBRIM confirms the long-term clinical benefit and safety profile of cobimetinib plus vemurafenib compared with vemurafenib monotherapy in patients with BRAFV600 mutation-positive advanced melanoma.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
- Clin Cancer Res. 27:5151.
- Clin Cancer Res. 27:5151.
References
-
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867–76. - PubMed
-
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–88. - PubMed
-
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–9. - PubMed
-
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381:626–36. - PubMed

