KL-VS heterozygosity is associated with lower amyloid-dependent tau accumulation and memory impairment in Alzheimer's disease
- PMID: 34158479
- PMCID: PMC8219708
- DOI: 10.1038/s41467-021-23755-z
KL-VS heterozygosity is associated with lower amyloid-dependent tau accumulation and memory impairment in Alzheimer's disease
Abstract
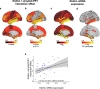
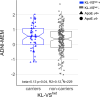
Klotho-VS heterozygosity (KL-VShet) is associated with reduced risk of Alzheimer's disease (AD). However, whether KL-VShet is associated with lower levels of pathologic tau, i.e., the key AD pathology driving neurodegeneration and cognitive decline, is unknown. Here, we assessed the interaction between KL-VShet and levels of beta-amyloid, a key driver of tau pathology, on the levels of PET-assessed neurofibrillary tau in 551 controls and patients across the AD continuum. KL-VShet showed lower cross-sectional and longitudinal increase in tau-PET per unit increase in amyloid-PET when compared to that of non-carriers. This association of KL-VShet on tau-PET was stronger in Klotho mRNA-expressing brain regions mapped onto a gene expression atlas. KL-VShet was related to better memory functions in amyloid-positive participants and this association was mediated by lower tau-PET. Amyloid-PET levels did not differ between KL-VShet carriers versus non-carriers. Together, our findings provide evidence to suggest a protective role of KL-VShet against amyloid-related tau pathology and tau-related memory impairments in elderly humans at risk of AD dementia.
Conflict of interest statement
M.B. received speaker honoraria from GE healthcare and LMI and is an advisor of LMI. All other authors declare no competing interests.
Figures