Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease
- PMID: 34158806
- PMCID: PMC8187052
- DOI: 10.1155/2021/9999146
Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease
Abstract
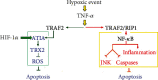
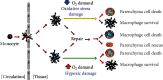
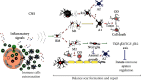
The concept of central nervous system (CNS) inflammation has evolved over the last decades. Neuroinflammation is the response of reactive CNS components to altered homeostasis, regardless of the cause to be endogenous or exogenous. Neurological diseases, whether traumatic, neoplastic, ischemic, metabolic, toxic, infectious, autoimmune, developmental, or degenerative, involve direct and indirect immune-related neuroinflammation. Brain infiltrates of the innate and adaptive immune system cells appear in response to an infective or otherwise noxious agent and produce inflammatory mediators. Mediators of inflammation include local and recruited cells and signals. Processes derived from extrinsic and intrinsic CNS diseases also elicit the CNS inflammatory response. A deeper understanding of immune-related inflammation in health and disease is necessary to find potential therapeutic targets for preventing or reducing CNS damage. This review is aimed at discussing the innate and adaptive immune system functions and their roles in regulating brain cell responses in disease and homeostasis maintenance.
Copyright © 2021 Rodolfo Kölliker-Frers et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

