Anti-biofilm activity of antibiotic-loaded Hylomate®
- PMID: 34159252
- PMCID: PMC8203729
- DOI: 10.1016/j.ijcha.2021.100801
Anti-biofilm activity of antibiotic-loaded Hylomate®
Abstract
Introduction: Antibiotic envelopes are being developed for cardiac implantable electronic device (CIED) wrapping to reduce the risk of infections.
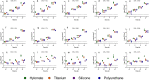
Methods: Fifteen CIED infection-associated bacterial isolates of Staphylococcus aureus, Staphylococcus epidermidis and Cutibacterium acnes were used to assess in vitro biofilm formation on Hylomate® compared to titanium, silicone and polyurethane coupons pre-treated with vancomycin (400 µg/ml), bacitracin (1000 U/ml) or a combination of rifampin (80 µg/ml) plus minocycline (50 µg/ml). Scanning electron microscopy (SEM) was performed to visualize bacteria on Hylomate®.
Results: There was significantly less (p < 0.05) S. aureus and S. epidermidis on Hylomate® pre-treated with vancomycin, bacitracin or rifampin plus minocycline after 24 h of incubation (≤1.00 log10 CFU/cm2) compared with titanium, silicone or polyurethane pre-treated with vancomycin, bacitracin or rifampin plus minocycline. C. acnes biofilms were not detected (≤1.00 log10 CFU/cm2) on pre-treated Hylomate® coupons.
Conclusions: This study showed that Hylomate® coupons pre-treated with antibiotics reduced staphylococcal and C. acnes biofilm formation in vitro.
Keywords: Antibacterial envelope; Biofilm; Cardiac device infection; Cutibacterium acnes; Staphylococcus aureus; Staphylococcus epidermidis.
© 2021 Mayo Clinic.
Conflict of interest statement
Dr. Patel reports grants from Merck, ContraFect, TenNor Therapeutics Limited, Hylomorph and Shionogi. Dr. Patel is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, and Qvella; monies are paid to Mayo Clinic. Dr. Patel is also a consultant to Netflix. In addition, Dr. Patel has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Dr. Patel receives an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course.
Figures




References
-
- Greenspon A.J., Patel J.D., Lau E., Ochoa J.A., Frisch D.R., Ho R.T. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J. Am. Coll. Cardiol. 2011;58:1001–1006. - PubMed
-
- Sohail M.R., Eby E.L., Ryan M.P., Gunnarsson C., Wright L.A., Greenspon A.J. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ: Arrhythmia Electrophysiol. 2016;9:e003929. - PubMed
-
- Greenspon A.J., Eby E.L., Petrilla A.A., Sohail M.R. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin. Electrophysiol. 2018;41:495–503. - PubMed
-
- Shariff N., Eby E., Adelstein E., Jain S., Shalaby A., Saba S. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J. Cardiovasc. Electrophysiol. 2015;26:783–789. - PubMed
LinkOut - more resources
Full Text Sources

