In vitro inhibitory activity against HPV of the monoterpenoid zinc tetra-ascorbo-camphorate
- PMID: 34159277
- PMCID: PMC8203719
- DOI: 10.1016/j.heliyon.2021.e07232
In vitro inhibitory activity against HPV of the monoterpenoid zinc tetra-ascorbo-camphorate
Abstract
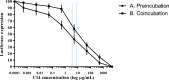
Zinc tetra-ascorbo-camphorate (or drug "C14") is a synthetic monoterpenoid derivative that has potent anti-HIV-1 activity in vitro. In this study, we evaluated the in vitro antiviral properties of C14 against human papillomavirus (HPV). Inhibition assay of HPV-16-pseudovirus (PsVs) adsorption on COS-7 cells by C14 was used. C14 inhibited HPV-16-PsVs adsorption with IC50 ranging between 2.9 and 8.3 μM and therapeutic indexes between >410 to >3,300. Pretreatment of COS-7 cells with C14 before addition of HPV-16-PsV was associated with more potent anti-HPV activity than simultaneous deposition on COS-7 of HPV-16-PsV and C14, suggesting that C14 is more effective in preventing HPV attachment to target cells than post-HPV adsorption viral events. Overall, these in vitro studies suggest that the monoterpenoid zinc tetra-ascorbo-camphorate molecule may be suitable for further clinical evaluations as potential microbicide or therapeutic drug.
Keywords: Camphor derivates; HPV; HPV-16; Inhibition assay; L-ascorbic acid conjugate; Pseudovirus; Terpenoid; Zn metal.
© 2021 The Author(s).
Conflict of interest statement
The authors declare the following conflict of interests: Ralph Sydney Mboumba Bouassa, Gabin Mwande-Maguene and Laurent Bélec report no conflicts of interest. Bernard Gombert, the chief executive officer of MGB Pharma, Nîmes, France, gave the C14 molecule for the study. Aurèle Mannarini is chemist adviser for MGB Pharma, and discussed the interpretation of the results. Bernard Gombert and Aurèle Mannarini did not play a role in the study design, data collection and analysis, as well as decision to publish.
Figures

References
-
- Scheurer M.E., Tortolero-Luna G., Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int. J. Gynecol. Canc. 2005 Sep-Oct;15(5):727–746. - PubMed
-
- Parkin D.M., Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine. 2006 Aug 31;24(Suppl 3:S3):11–25. - PubMed
-
- World health Organization . 2015. Projections of Mortality and Causes of Death, 2015 and 2030.http://www.who.int/healthinfo/global_burden_disease/projections/en/ Available at:
-
- Mboumba Bouassa R.S., Prazuck T., Lethu T., Jenabian M.A., Meye J.F., Bélec L. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. Expert Rev. Anti Infect. Ther. 2017 Jun;15(6):613–627. - PubMed
-
- Harper D.M., DeMars L.R. HPV vaccines - a review of the first decade. Gynecol. Oncol. 2017 Jul;146(1):196–204. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

