Generating CAR T cells from tumor-infiltrating lymphocytes
- PMID: 34159293
- PMCID: PMC8186112
- DOI: 10.1177/25151355211017119
Generating CAR T cells from tumor-infiltrating lymphocytes
Abstract
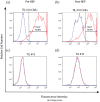
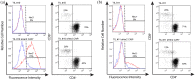
Background: Tumor-infiltrating lymphocytes (TILs) and chimeric antigen receptor (CAR) T-cell therapies have demonstrated promising, though limited, efficacy against melanoma. Methods: We designed a model system to explore the efficacy of dual specific T cells derived from melanoma patient TILs by transduction with a Her2-specific CAR. Results: Metastatic melanoma cells in our biobank constitutively expressed Her2 antigen. CAR-TIL produced greater amounts of IFN compared with parental TIL, when co-cultured with Her2 expressing tumor lines, including autologous melanoma tumor lines, although no consistent increase in cytotoxicity by TIL was afforded by expression of a CAR. Results of an in vivo study in NSG mice demonstrated tumor shrinkage when CAR-TILs were used in an adoptive cell therapy protocol. Conclusion: Potential limitations of transduced TIL in our study included limited proliferative potential and a terminally differentiated phenotype, which would need addressing in further work before consideration of clinical translation.
Keywords: Her2; autologous tumor; chimeric antigen receptor; immunotherapy; melanoma.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures






References
-
- Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012; 72: 2746–2756. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

