This is a preprint.
Structures of synthetic nanobody-SARS-CoV-2-RBD complexes reveal distinct sites of interaction and recognition of variants
- PMID: 34159326
- PMCID: PMC8219104
- DOI: 10.21203/rs.3.rs-625642/v1
Structures of synthetic nanobody-SARS-CoV-2-RBD complexes reveal distinct sites of interaction and recognition of variants
Update in
-
Structures of synthetic nanobody-SARS-CoV-2 receptor-binding domain complexes reveal distinct sites of interaction.J Biol Chem. 2021 Oct;297(4):101202. doi: 10.1016/j.jbc.2021.101202. Epub 2021 Sep 16. J Biol Chem. 2021. PMID: 34537245 Free PMC article.
Abstract
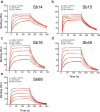
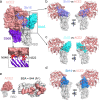
The worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and emergence of new variants demands understanding the structural basis of the interaction of antibodies with the SARS-CoV-2 receptor-binding domain (RBD). Here we report five X-ray crystal structures of sybodies (synthetic nanobodies) including binary and ternary complexes of Sb16-RBD, Sb45-RBD, Sb14-RBD-Sb68, and Sb45-RBD-Sb68; and Sb16 unliganded. These reveal that Sb14, Sb16, and Sb45 bind the RBD at the ACE2 interface and that the Sb16 interaction is accompanied by a large CDR2 shift. In contrast, Sb68 interacts at the periphery of the interface. We also determined cryo-EM structures of Sb45 bound to spike (S). Superposition of the X-ray structures of sybodies onto the trimeric S protein cryo-EM map indicates some may bind both "up" and "down" configurations, but others may not. Sensitivity of sybody binding to several recently identified RBD mutants is consistent with these structures.
Conflict of interest statement
Figures




References
-
- Conti P. et al. The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J Biol Regul Homeost Agents 35(2021). - PubMed
-
- Wibmer C.K. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv, 2021.01.18.427166 (2021). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

