This is a preprint.
Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention
- PMID: 34159344
- PMCID: PMC8219114
- DOI: 10.1101/2021.06.14.21258567
Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention
Update in
-
Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19.N Engl J Med. 2021 Sep 23;385(13):1184-1195. doi: 10.1056/NEJMoa2109682. Epub 2021 Aug 4. N Engl J Med. 2021. PMID: 34347950 Free PMC article. Clinical Trial.
Abstract
Background: Casirivimab and imdevimab (REGEN-COV™) markedly reduces risk of hospitalization or death in high-risk individuals with Covid-19. Here we explore the possibility that subcutaneous REGEN-COV prevents SARS-CoV-2 infection and subsequent Covid-19 in individuals at high risk of contracting SARS-CoV-2 by close exposure in a household with a documented SARS-CoV-2-infected individual.
Methods: Individuals ≥12 years were enrolled within 96 hours of a household contact being diagnosed with SARS-CoV-2 and randomized 1:1 to receive 1200 mg REGEN-COV or placebo via subcutaneous injection. The primary efficacy endpoint was the proportion of participants without evidence of infection (SARS-CoV-2 RT-qPCR-negative) or prior immunity (seronegative) who subsequently developed symptomatic SARS-CoV-2 infection during a 28-day efficacy assessment period.
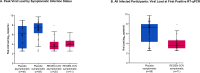
Results: Subcutaneous REGEN-COV significantly prevented symptomatic SARS-CoV-2 infection compared with placebo (81.4% risk reduction; 11/753 [1.5%] vs. 59/752 [7.8%], respectively; P<0.0001), with 92.6% risk reduction after the first week (2/753 [0.3%] vs. 27/752 [3.6%], respectively). REGEN-COV also prevented overall infections, either symptomatic or asymptomatic (66.4% risk reduction). Among infected participants, the median time to resolution of symptoms was 2 weeks shorter with REGEN-COV vs. placebo (1.2 vs. 3.2 weeks, respectively), and the duration of time with high viral load (>104 copies/mL) was lower (0.4 vs. 1.3 weeks, respectively). REGEN-COV was generally well tolerated.
Conclusions: Administration of subcutaneous REGEN-COV prevented symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection in uninfected household contacts of infected individuals. Among individuals who became infected, REGEN-COV reduced the duration of symptomatic disease, decreased maximal viral load, and reduced the duration of detectable virus.(ClinicalTrials.gov number, NCT04452318.).
Figures




References
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. 2020. at https://www.who.int/director-general/speeches/detail/who-director-genera....)
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
