Carborane Stabilized "19-Electron" Molybdenum Metalloradical
- PMID: 34160218
- PMCID: PMC8397321
- DOI: 10.1021/jacs.1c03568
Carborane Stabilized "19-Electron" Molybdenum Metalloradical
Abstract
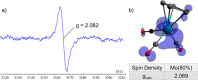
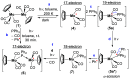
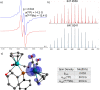
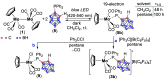
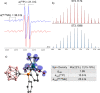
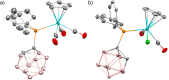
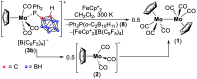
Paramagnetic metal complexes gained a lot of attention due to their participation in a number of important chemical reactions. In most cases, these complexes are dominated by 17-e metalloradicals that are associatively activated with highly reactive paramagnetic 19-e species. Molybdenum paramagnetic complexes are among the most investigated ones. While some examples of persistent 17-e Mo-centered radicals have been reported, in contrast, 19-e Mo-centered radicals are illusive species and as such could rarely be detected. In this work, the photodissociation of the [Cp(CO)3Mo]2 dimer (1) in the presence of phosphines was revisited. As a result, the first persistent, formally 19-e Mo radical with significant electron density on the Mo center (22%), Cp(CO)3Mo•PPh2(o-C2B10H11) (5b), was generated and characterized by EPR spectroscopy and MS as well as studied by DFT calculations. The stabilization of 5b was likely achieved due to a unique electron-withdrawing effect of the o-carboranyl substituent at the phosphorus center.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tolman C. A. The 16 and 18 Electron Rule in Organometallic Chemistry and Homogeneous Catalysis. Chem. Soc. Rev. 1972, 1, 337–353. 10.1039/cs9720100337. - DOI
-
- Elschenbroich C.; Salzer A.. Organometallics: A Concise Introduction, 1st ed.; VCH: Weinheim, 1989.
-
- Kochi J. K.Preface. In Organometallic Mechanisms and Catalysis; Kochi J. K., Ed.; Academic Press: 1978; pp xiii–xiv.
-
- Astruc D. Nineteen-Electron Complexes and Their Role in Organometallic Mechanisms. Chem. Rev. 1988, 88, 1189–1216. 10.1021/cr00089a010. - DOI
-
- Song J. S.; Bullock R. M.; Creutz C. Intrinsic Barriers to Atom Transfer (Abstraction) Processes; Self-Exchange Rates for Cp(CO)3M• Radical/Cp(CO)3M-X Halogen Couples. J. Am. Chem. Soc. 1991, 113, 9862–9864. 10.1021/ja00026a029. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

