Association of Diverse Staphylococcus aureus Populations with Pseudomonas aeruginosa Coinfection and Inflammation in Cystic Fibrosis Airway Infection
- PMID: 34160233
- PMCID: PMC8265651
- DOI: 10.1128/mSphere.00358-21
Association of Diverse Staphylococcus aureus Populations with Pseudomonas aeruginosa Coinfection and Inflammation in Cystic Fibrosis Airway Infection
Abstract
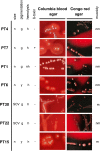
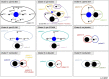
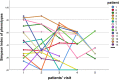
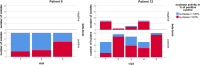
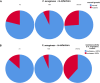
Staphylococcus aureus is one of the most common pathogens isolated from the airways of cystic fibrosis (CF) patients and often persists for extended periods. There is limited knowledge about the diversity of S. aureus in CF. We hypothesized that increased diversity of S. aureus would impact CF lung disease. Therefore, we conducted a 1-year observational prospective study with 14 patients with long-term S. aureus infection. From every sputum, 40 S. aureus isolates were chosen and characterized in terms of phenotypic appearance (size, hemolysis, mucoidy, and pigmentation), important virulence traits such as nuclease activity, biofilm formation, and molecular typing by spa sequence typing. Data about coinfection with Pseudomonas aeruginosa and clinical parameters such as lung function, exacerbation, and inflammatory markers in blood (C-reactive protein [CRP], interleukin 6 [IL-6], and S100A8/9 [calprotectin]) were collected. From 58 visits of 14 patients, 2,319 S. aureus isolates were distinguished into 32 phenotypes (PTs) and 50 spa types. The Simpson diversity index (SDI) was used to calculate the phenotypic and genotypic diversity, revealing a high diversity of PTs ranging from 0.19 to 0.87 among patients, while the diversity of spa types of isolates was less pronounced. The SDI of PTs was positively associated with P. aeruginosa coinfection and inflammatory parameters, with IL-6 being the most sensitive parameter. Also, coinfection with P. aeruginosa was associated with mucoid S. aureus and S. aureus with high nuclease activity. Our analyses showed that in CF patients with long-term S. aureus airway infection, a highly diverse and dynamic S. aureus population was present and associated with P. aeruginosa coinfection and inflammation. IMPORTANCE Staphylococcus aureus can persist for extended periods in the airways of people with cystic fibrosis (CF) in spite of antibiotic therapy and high numbers of neutrophils, which fail to eradicate this pathogen. Therefore, S. aureus needs to adapt to this hostile niche. There is only limited knowledge about the diversity of S. aureus in respiratory specimens. We conducted a 1-year prospective study with 14 patients with long-term S. aureus infection and investigated 40 S. aureus isolates from every sputum in terms of phenotypic appearance, nuclease activity, biofilm formation, and molecular typing. Data about coinfection with Pseudomonas aeruginosa and clinical parameters such as lung function, exacerbation, and inflammatory markers in blood were collected. Thirty-two phenotypes (PTs) and 50 spa types were distinguished. Our analyses revealed that in CF patients with long-term S. aureus airway infection, a highly diverse and dynamic S. aureus population was associated with P. aeruginosa coinfection and inflammation.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; cystic fibrosis; diversity; persistent infection.
Figures





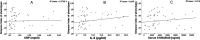
Similar articles
-
Genotypic and Phenotypic Diversity of Staphylococcus aureus Isolates from Cystic Fibrosis Patient Lung Infections and Their Interactions with Pseudomonas aeruginosa.mBio. 2020 Jun 23;11(3):e00735-20. doi: 10.1128/mBio.00735-20. mBio. 2020. PMID: 32576671 Free PMC article.
-
Staphylococcus aureus in the airways of cystic fibrosis patients - A retrospective long-term study.Int J Med Microbiol. 2018 Aug;308(6):631-639. doi: 10.1016/j.ijmm.2018.02.003. Epub 2018 Feb 24. Int J Med Microbiol. 2018. PMID: 29501453
-
Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection.mSphere. 2018 Aug 15;3(4):e00341-18. doi: 10.1128/mSphere.00341-18. mSphere. 2018. PMID: 30111629 Free PMC article.
-
Pulmonary Pathogens Adapt to Immune Signaling Metabolites in the Airway.Front Immunol. 2020 Mar 13;11:385. doi: 10.3389/fimmu.2020.00385. eCollection 2020. Front Immunol. 2020. PMID: 32231665 Free PMC article. Review.
-
Microbiology of airway disease in patients with cystic fibrosis.Clin Microbiol Rev. 1991 Jan;4(1):35-51. doi: 10.1128/CMR.4.1.35. Clin Microbiol Rev. 1991. PMID: 1900735 Free PMC article. Review.
Cited by
-
Quorum sensing and antibiotic resistance in polymicrobial infections.Commun Integr Biol. 2024 Oct 17;17(1):2415598. doi: 10.1080/19420889.2024.2415598. eCollection 2024. Commun Integr Biol. 2024. PMID: 39430726 Free PMC article. Review.
-
Staphylococcus aureus and Pseudomonas aeruginosa Isolates from the Same Cystic Fibrosis Respiratory Sample Coexist in Coculture.Microbiol Spectr. 2022 Aug 31;10(4):e0097622. doi: 10.1128/spectrum.00976-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867391 Free PMC article.
-
Anti-Biofilm Activity of Carnosic Acid from Salvia rosmarinus against Methicillin-Resistant Staphylococcus aureus.Plants (Basel). 2023 Oct 25;12(21):3679. doi: 10.3390/plants12213679. Plants (Basel). 2023. PMID: 37960038 Free PMC article.
-
Staphylococcus aureus Small-Colony Variants from Airways of Adult Cystic Fibrosis Patients as Precursors of Adaptive Antibiotic-Resistant Mutations.Antibiotics (Basel). 2023 Jun 17;12(6):1069. doi: 10.3390/antibiotics12061069. Antibiotics (Basel). 2023. PMID: 37370388 Free PMC article.
-
Biofilm Formation in Methicillin-Resistant Staphylococcus aureus Isolated in Cystic Fibrosis Patients Is Strain-Dependent and Differentially Influenced by Antibiotics.Front Microbiol. 2021 Oct 15;12:750489. doi: 10.3389/fmicb.2021.750489. eCollection 2021. Front Microbiol. 2021. PMID: 34721354 Free PMC article.
References
-
- Cystic Fibrosis Foundation. 2018. 2017 Patient Registry Annual Data Report. Cystic Fibrosis Foundation, Bethesda, Maryland.
-
- Hatziagorou E, Orenti A, Drevinek P, Kashirskaya N, Mei-Zahav M, de Boeck K, ECFSPR . 2020. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis–data from the European Cystic Fibrosis Society Patient Registry. J Cyst Fibros 19:376–383. doi:10.1016/j.jcf.2019.08.006. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

