Limitation of Microbial Processes at Saturation-Level Salinities in a Microbial Mat Covering a Coastal Salt Flat
- PMID: 34160273
- PMCID: PMC8357274
- DOI: 10.1128/AEM.00698-21
Limitation of Microbial Processes at Saturation-Level Salinities in a Microbial Mat Covering a Coastal Salt Flat
Abstract
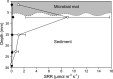
Hypersaline microbial mats are dense microbial ecosystems capable of performing complete element cycling and are considered analogs of early Earth and hypothetical extraterrestrial ecosystems. We studied the functionality and limits of key biogeochemical processes, such as photosynthesis, aerobic respiration, and sulfur cycling, in salt crust-covered microbial mats from a tidal flat at the coast of Oman. We measured light, oxygen, and sulfide microprofiles as well as sulfate reduction rates at salt saturation and in flood conditions and determined fine-scale stratification of pigments, biomass, and microbial taxa in the resident microbial community. The salt crust did not protect the mats against irradiation or evaporation. Although some oxygen production was measurable at salinities of ≤30% (wt/vol) in situ, at saturation-level salinity (40%), oxygenic photosynthesis was completely inhibited and only resumed 2 days after reducing the porewater salinity to 12%. Aerobic respiration and active sulfur cycling occurred at low rates under salt saturation and increased strongly upon salinity reduction. Apart from high relative abundances of Chloroflexi, photoheterotrophic Alphaproteobacteria, Bacteroidetes, and Archaea, the mat contained a distinct layer harboring filamentous Cyanobacteria, which is unusual for such high salinities. Our results show that the diverse microbial community inhabiting this salt flat mat ultimately depends on periodic salt dilution to be self-sustaining and is rather adapted to merely survive salt saturation than to thrive under the salt crust. IMPORTANCE Due to their abilities to survive intense radiation and low water availability, hypersaline microbial mats are often suggested to be analogs of potential extraterrestrial life. However, even the limitations imposed on microbial processes by saturation-level salinity found on Earth have rarely been studied in situ. While abundance and diversity of microbial life in salt-saturated environments are well documented, most of our knowledge on process limitations stems from culture-based studies, few in situ studies, and theoretical calculations. In particular, oxygenic photosynthesis has barely been explored beyond 5 M NaCl (28% wt/vol). By applying a variety of biogeochemical and molecular methods, we show that despite abundance of photoautotrophic microorganisms, oxygenic photosynthesis is inhibited in salt-crust-covered microbial mats at saturation salinities, while rates of other energy generation processes are decreased several-fold. Hence, the complete element cycling required for self-sustaining microbial communities only occurs at lower salt concentrations.
Keywords: biofilm biology; element cycles and biogeochemical processes; extremophiles/extremophily; microbial communities; microbiology of unexplored habitats; primary and secondary production; uncultured microbes.
Figures






References
-
- O'Malley-James JT, Greaves JS, Raven JA, Cockell CS. 2013. Swansong biospheres: refuges for life and novel microbial biospheres on terrestrial planets near the end of their habitable lifetimes. Int J Astrobiol 12:99–112. 10.1017/S147355041200047X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

