Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial
- PMID: 34160577
- PMCID: PMC8223138
- DOI: 10.1001/jamasurg.2021.2373
Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial
Erratum in
-
Errors in a Figure.JAMA Surg. 2022 Sep 1;157(9):859. doi: 10.1001/jamasurg.2022.3765. JAMA Surg. 2022. PMID: 35921117 Free PMC article. No abstract available.
Abstract
Importance: The prognosis of patients with locally advanced esophageal squamous cell carcinoma (ESCC) remains poor after surgery. Neoadjuvant chemoradiotherapy (NCRT) has been shown to potentially improve survival.
Objective: To compare the treatment efficacy of NCRT plus surgery with surgery alone for long-term survival among patients with locally advanced ESCC.
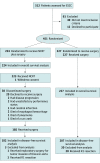
Design, setting, and participants: The Neoadjuvant Chemoradiotherapy for Esophageal Cancer 5010 study was a multicenter open-label randomized phase 3 clinical trial that enrolled patients between June 1, 2007, and December 31, 2014. Follow-up ended on December 31, 2019. The study was conducted at 8 centers in China. A total of 451 patients aged 18 to 70 years with thoracic ESCC stage T1-4N1M0/T4N0M0 were enrolled and randomized. Data were analyzed from December 1, 2019, to June 30, 2020.
Interventions: Patients randomized to receive NCRT plus surgery (NCRT group) received preoperative chemotherapy (25 mg/m2 of vinorelbine on days 1 and 8 and 75 mg/m2 of cisplatin on day 1 or 25 mg/m2 of cisplatin on days 1 to 4) every 3 weeks for 2 cycles and concurrent radiotherapy (40.0 Gy, administered in 20 fractions of 2.0 Gy for 5 days per week) followed by surgery. Patients randomized to receive surgery alone (surgery group) underwent surgery after randomization.
Main outcomes and measures: The primary end point was overall survival in the intention-to-treat population. The secondary end point was disease-free survival.
Results: A total of 451 patients (mean [SD] age, 56.5 [7.0] years; 367 men [81.4%]) were randomized to the NCRT (n = 224) and surgery (n = 227) groups and were eligible for the intention-to-treat analysis. By December 31, 2019, 224 deaths had occurred. The median follow-up was 53.5 months (interquartile range, 18.2-87.4 months). Patients receiving NCRT plus surgery had prolonged overall survival compared with those receiving surgery alone (hazard ratio, 0.74; 95% CI, 0.57-0.97; P = .03), with a 5-year survival rate of 59.9% (95% CI, 52.9%-66.1%) vs 49.1% (95% CI, 42.3%-55.6%), respectively. Patients in the NCRT group compared with the surgery group also had prolonged disease-free survival (hazard ratio, 0.60; 95% CI, 0.45-0.80; P < .001), with a 5-year survival rate of 63.6% (95% CI, 56.0%-70.2%) vs 43.0% (95% CI, 36.0%-49.7%), respectively.
Conclusions and relevance: In this randomized clinical trial, treatment with NCRT plus surgery significantly improved long-term overall survival and disease-free survival and therefore may be considered a standard of care for patients with locally advanced ESCC.
Trial registration: ClinicalTrials.gov Identifier: NCT01216527.
Conflict of interest statement
Figures


Comment in
-
When East Meets West, Understanding Is a 2-Way Street.JAMA Surg. 2021 Aug 1;156(8):729-730. doi: 10.1001/jamasurg.2021.2374. JAMA Surg. 2021. PMID: 34160583 No abstract available.
-
Evaluating Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma.JAMA Surg. 2022 May 1;157(5):458-459. doi: 10.1001/jamasurg.2021.7113. JAMA Surg. 2022. PMID: 35080625 No abstract available.
-
Evaluating Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma-Reply.JAMA Surg. 2022 May 1;157(5):459-460. doi: 10.1001/jamasurg.2021.7114. JAMA Surg. 2022. PMID: 35080629 No abstract available.
References
-
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. ; CROSS Study Group . Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090-1098. doi:10.1016/S1470-2045(15)00040-6 - DOI - PubMed
-
- Sjoquist KM, Burmeister BH, Smithers BM, et al. ; Australasian Gastro-Intestinal Trials Group . Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681-692. doi:10.1016/S1470-2045(11)70142-5 - DOI - PubMed

