Risk Prediction Model of 90-Day Mortality After Esophagectomy for Cancer
- PMID: 34160587
- PMCID: PMC8223144
- DOI: 10.1001/jamasurg.2021.2376
Risk Prediction Model of 90-Day Mortality After Esophagectomy for Cancer
Erratum in
-
Error in a Supplement.JAMA Surg. 2021 Sep 1;156(9):894. doi: 10.1001/jamasurg.2021.4340. JAMA Surg. 2021. PMID: 34495318 Free PMC article. No abstract available.
Abstract
Importance: Ninety-day mortality rates after esophagectomy are an indicator of the quality of surgical oncologic management. Accurate risk prediction based on large data sets may aid patients and surgeons in making informed decisions.
Objective: To develop and validate a risk prediction model of death within 90 days after esophagectomy for cancer using the International Esodata Study Group (IESG) database, the largest existing prospective, multicenter cohort reporting standardized postoperative outcomes.
Design, setting, and participants: In this diagnostic/prognostic study, we performed a retrospective analysis of patients from 39 institutions in 19 countries between January 1, 2015, and December 31, 2019. Patients with esophageal cancer were randomly assigned to development and validation cohorts. A scoring system that predicted death within 90 days based on logistic regression β coefficients was conducted. A final prognostic score was determined and categorized into homogeneous risk groups that predicted death within 90 days. Calibration and discrimination tests were assessed between cohorts.
Exposures: Esophageal resection for cancer of the esophagus and gastroesophageal junction.
Main outcomes and measures: All-cause postoperative 90-day mortality.
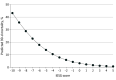
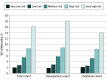
Results: A total of 8403 patients (mean [SD] age, 63.6 [9.0] years; 6641 [79.0%] male) were included. The 30-day mortality rate was 2.0% (n = 164), and the 90-day mortality rate was 4.2% (n = 353). Development (n = 4172) and validation (n = 4231) cohorts were randomly assigned. The multiple logistic regression model identified 10 weighted point variables factored into the prognostic score: age, sex, body mass index, performance status, myocardial infarction, connective tissue disease, peripheral vascular disease, liver disease, neoadjuvant treatment, and hospital volume. The prognostic scores were categorized into 5 risk groups: very low risk (score, ≥1; 90-day mortality, 1.8%), low risk (score, 0; 90-day mortality, 3.0%), medium risk (score, -1 to -2; 90-day mortality, 5.8%), high risk (score, -3 to -4: 90-day mortality, 8.9%), and very high risk (score, ≤-5; 90-day mortality, 18.2%). The model was supported by nonsignificance in the Hosmer-Lemeshow test. The discrimination (area under the receiver operating characteristic curve) was 0.68 (95% CI, 0.64-0.72) in the development cohort and 0.64 (95% CI, 0.60-0.69) in the validation cohort.
Conclusions and relevance: In this study, on the basis of preoperative variables, the IESG risk prediction model allowed stratification of an individual patient's risk of death within 90 days after esophagectomy. These data suggest that this model can help in the decision-making process when esophageal cancer surgery is being considered and in informed consent.
Conflict of interest statement
Figures
Comment in
-
Predicting Mortality Rates After Esophagectomy for Cancer.JAMA Surg. 2021 Sep 1;156(9):845-846. doi: 10.1001/jamasurg.2021.2377. JAMA Surg. 2021. PMID: 34160591 No abstract available.
-
Questions About a Risk Prediction Model of Mortality After Esophagectomy for Cancer.JAMA Surg. 2022 Mar 1;157(3):279. doi: 10.1001/jamasurg.2021.5698. JAMA Surg. 2022. PMID: 34730796 No abstract available.
-
Questions About a Risk Prediction Model of Mortality After Esophagectomy for Cancer.JAMA Surg. 2022 Mar 1;157(3):279-280. doi: 10.1001/jamasurg.2021.5701. JAMA Surg. 2022. PMID: 34730807 No abstract available.
References
-
- van der Sluis PC, van der Horst S, May AM, et al. . Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg. 2019;269(4):621-630. doi:10.1097/SLA.0000000000003031 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

