Nanoparticle-Cartilage Interaction: Pathology-Based Intra-articular Drug Delivery for Osteoarthritis Therapy
- PMID: 34160733
- PMCID: PMC8222488
- DOI: 10.1007/s40820-021-00670-y
Nanoparticle-Cartilage Interaction: Pathology-Based Intra-articular Drug Delivery for Osteoarthritis Therapy
Abstract
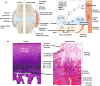
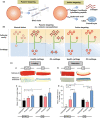
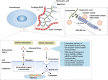
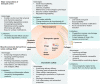
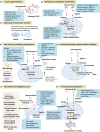
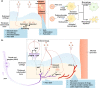
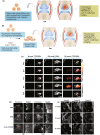
Osteoarthritis is the most prevalent chronic and debilitating joint disease, resulting in huge medical and socioeconomic burdens. Intra-articular administration of agents is clinically used for pain management. However, the effectiveness is inapparent caused by the rapid clearance of agents. To overcome this issue, nanoparticles as delivery systems hold considerable promise for local control of the pharmacokinetics of therapeutic agents. Given the therapeutic programs are inseparable from pathological progress of osteoarthritis, an ideal delivery system should allow the release of therapeutic agents upon specific features of disorders. In this review, we firstly introduce the pathological features of osteoarthritis and the design concept for accurate localization within cartilage for sustained drug release. Then, we review the interactions of nanoparticles with cartilage microenvironment and the rational design. Furthermore, we highlight advances in the therapeutic schemes according to the pathology signals. Finally, armed with an updated understanding of the pathological mechanisms, we place an emphasis on the development of "smart" bioresponsive and multiple modality nanoparticles on the near horizon to interact with the pathological signals. We anticipate that the exploration of nanoparticles by balancing the efficacy, safety, and complexity will lay down a solid foundation tangible for clinical translation.
Keywords: Articular cartilage; Drug delivery; Nanomedicine; Nanoparticle; Osteoarthritis.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
