Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective
- PMID: 34161185
- PMCID: PMC8526011
- DOI: 10.1080/15548627.2021.1938914
Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective
Abstract
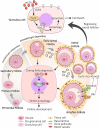
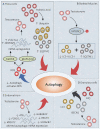
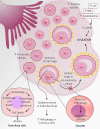
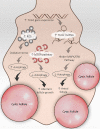
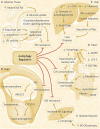
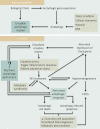
Polycystic ovary syndrome (PCOS) is a unification of endocrine and metabolic disorders and has become immensely prevalent among women of fertile age. The prime organ affected in PCOS is the ovary and its distressed functioning elicits disturbed reproductive outcomes. In the ovary, macroautophagy/autophagy performs a pivotal role in directing the chain of events starting from oocytes origin until its fertilization. Recent discoveries demonstrate a significant role of autophagy in the pathogenesis of PCOS. Defective autophagy in the follicular cells during different stages of follicles is observed in the PCOS ovary. Exploring different autophagy pathways provides a platform for predicting the possible cause of altered ovarian physiology in PCOS. In this review, we have emphasized autophagy's role in governing follicular development under normal circumstances and in PCOS, including significant abnormalities associated with PCOS such as anovulation, hyperandrogenemia, metabolic disturbances, and related abnormality. So far, few studies have linked autophagy and PCOS and propose its essential role in PCOS progression. However, detailed knowledge in this area is lacking. Here we have summarized the latest knowledge related to autophagy associated with PCOS. This review's main objective is to provide a background of autophagy in the ovary, its possible connection with PCOS and suggested a novel proposal for future studies to aid a better understanding of PCOS pathogenesis.Abbreviations: AE: androgen excess; AF: antral follicle; AKT/PKB: AKT serine/threonine kinase; AMH: anti-Mullerian hormone; AMPK: AMP-activated protein kinase; ATG: autophagy-related; BCL2: BCL2 apoptosis regulator; BECN1: beclin 1; BMP: bone morphogenetic protein; CASP3: caspase 3; CL: corpus luteum; CYP17A1/P450C17: cytochrome P450 family 17 subfamily A member 1; CYP19A1: cytochrome P450 family 19 subfamily A member 1; DHEA: dehydroepiandrosterone; EH: endometrial hyperplasia; FF: follicular fluid; FOXO: forkhead box O; FSH: follicle stimulating hormone; GC: granulosa cell; GDF: growth differentiation factor; HA: hyperandrogenemia; HMGB1: high mobility group box 1; IGF1: insulin like growth factor 1; INS: insulin; IR: insulin resistance; LHCGR/LHR: luteinizing hormone/choriogonadotropin receptor; MAP1LC3B/LC3B: microtubule associated protein 1 light chain 3 beta; MAPK/ERK: mitogen-activated protein kinase; MAPK8/JNK: mitogen-activated protein kinase 8; MTOR: mechanistic target of rapamycin kinase; MTORC: mechanistic target of rapamycin complex; NAFLD: nonalcoholic fatty liver disease; NFKB: nuclear factor kappa B; OLR1/LOX-1: oxidized low density lipoprotein receptor 1; oxLDL: oxidized low-density lipoproteins; PA: palmitic acid; PCOS: polycystic ovary syndrome; PF: primary follicle; PGC: primordial germ cell; PI3K: phosphoinositide 3-kinase; PMF: primordial follicle; ROS: reactive oxygen species; RP: resting pool; SIRT1: sirtuin 1; SQSTM1/p62: sequestosome 1; T2DM: type 2 diabetes mellitus; TC: theca cell; TUG1: taurine up-regulated 1.
Keywords: Autophagy; LC3; autophagy-related proteins; beclin 1; follicle development; follicular atresia; granulosa cells death; polycystic ovary syndrome.
Figures




References
-
- Goodman NF, Cobin RH, Futterweit W, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - Part 1. Endocr Pract. 2015;21(11):1291–1300. - PubMed
-
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. - PubMed
-
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(2):181–191.
-
- Goodarzi MO, Dumesic DA, Chazenbalk G, et al. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. - PubMed
-
- Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
