Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis
- PMID: 34161316
- PMCID: PMC8221784
- DOI: 10.1371/journal.pone.0252617
Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis
Abstract
Background: Many studies report the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies. We aimed to synthesize seroprevalence data to better estimate the level and distribution of SARS-CoV-2 infection, identify high-risk groups, and inform public health decision making.
Methods: In this systematic review and meta-analysis, we searched publication databases, preprint servers, and grey literature sources for seroepidemiological study reports, from January 1, 2020 to December 31, 2020. We included studies that reported a sample size, study date, location, and seroprevalence estimate. We corrected estimates for imperfect test accuracy with Bayesian measurement error models, conducted meta-analysis to identify demographic differences in the prevalence of SARS-CoV-2 antibodies, and meta-regression to identify study-level factors associated with seroprevalence. We compared region-specific seroprevalence data to confirmed cumulative incidence. PROSPERO: CRD42020183634.
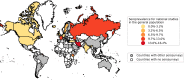
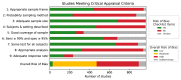
Results: We identified 968 seroprevalence studies including 9.3 million participants in 74 countries. There were 472 studies (49%) at low or moderate risk of bias. Seroprevalence was low in the general population (median 4.5%, IQR 2.4-8.4%); however, it varied widely in specific populations from low (0.6% perinatal) to high (59% persons in assisted living and long-term care facilities). Median seroprevalence also varied by Global Burden of Disease region, from 0.6% in Southeast Asia, East Asia and Oceania to 19.5% in Sub-Saharan Africa (p<0.001). National studies had lower seroprevalence estimates than regional and local studies (p<0.001). Compared to Caucasian persons, Black persons (prevalence ratio [RR] 3.37, 95% CI 2.64-4.29), Asian persons (RR 2.47, 95% CI 1.96-3.11), Indigenous persons (RR 5.47, 95% CI 1.01-32.6), and multi-racial persons (RR 1.89, 95% CI 1.60-2.24) were more likely to be seropositive. Seroprevalence was higher among people ages 18-64 compared to 65 and over (RR 1.27, 95% CI 1.11-1.45). Health care workers in contact with infected persons had a 2.10 times (95% CI 1.28-3.44) higher risk compared to health care workers without known contact. There was no difference in seroprevalence between sex groups. Seroprevalence estimates from national studies were a median 18.1 times (IQR 5.9-38.7) higher than the corresponding SARS-CoV-2 cumulative incidence, but there was large variation between Global Burden of Disease regions from 6.7 in South Asia to 602.5 in Sub-Saharan Africa. Notable methodological limitations of serosurveys included absent reporting of test information, no statistical correction for demographics or test sensitivity and specificity, use of non-probability sampling and use of non-representative sample frames.
Discussion: Most of the population remains susceptible to SARS-CoV-2 infection. Public health measures must be improved to protect disproportionately affected groups, including racial and ethnic minorities, until vaccine-derived herd immunity is achieved. Improvements in serosurvey design and reporting are needed for ongoing monitoring of infection prevalence and the pandemic response.
Conflict of interest statement
MPC reports personal fees from Gen1E Lifesciences (as a member of the scientific advisory board) and personal fees from nplex biosciences (as a member of the scientific advisory board), both outside the submitted work. JP reports grants and personal fees from Seegene and AbbVie, grants from MedImmune and Sanofi Pasteur, outside the submitted work. RKA, NB, and TY report grants from the World Health Organization and the Canadian Medical Association for SARS-CoV-2 serosurveillance, both outside the submitted work. DAC reports personal fees from Biobeats (https://www.bio-beat.com/), and Sensyne Health during the conduct of the study. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies.PLoS Med. 2022 Nov 10;19(11):e1004107. doi: 10.1371/journal.pmed.1004107. eCollection 2022 Nov. PLoS Med. 2022. PMID: 36355774 Free PMC article.
-
Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis.J Hosp Infect. 2021 Feb;108:120-134. doi: 10.1016/j.jhin.2020.11.008. Epub 2020 Nov 16. J Hosp Infect. 2021. PMID: 33212126 Free PMC article.
-
Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020-May 2021.JAMA. 2021 Oct 12;326(14):1400-1409. doi: 10.1001/jama.2021.15161. JAMA. 2021. PMID: 34473201 Free PMC article.
-
Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis.Lancet. 2022 Jun 25;399(10344):2351-2380. doi: 10.1016/S0140-6736(22)00484-6. Epub 2022 Apr 8. Lancet. 2022. PMID: 35405084 Free PMC article.
-
SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis.Clin Microbiol Infect. 2021 Mar;27(3):331-340. doi: 10.1016/j.cmi.2020.10.020. Epub 2020 Oct 24. Clin Microbiol Infect. 2021. PMID: 33228974 Free PMC article.
Cited by
-
Seroprevalence of SARS-CoV-2 in Bhubaneswar, India: findings from three rounds of community surveys.Epidemiol Infect. 2021 Apr 27;149:e139. doi: 10.1017/S0950268821000972. Epidemiol Infect. 2021. PMID: 33902776 Free PMC article.
-
Occupation and SARS-CoV-2 seroprevalence studies: a systematic review.BMJ Open. 2023 Feb 28;13(2):e063771. doi: 10.1136/bmjopen-2022-063771. BMJ Open. 2023. PMID: 36854599 Free PMC article.
-
Nigeria healthcare worker SARS-CoV-2 serology study: Results from a prospective, longitudinal cohort.PLOS Glob Public Health. 2023 Jan 17;3(1):e0000549. doi: 10.1371/journal.pgph.0000549. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 36962953 Free PMC article.
-
SARS-CoV-2 serosurvey among adults involved in healthcare and health research in Guinea-Bissau, West Africa.Public Health. 2022 Feb;203:19-22. doi: 10.1016/j.puhe.2021.11.013. Epub 2021 Dec 3. Public Health. 2022. PMID: 35016071 Free PMC article.
-
Temporal trends in disparities in COVID-19 seropositivity among Canadian blood donors.Int J Epidemiol. 2024 Apr 11;53(3):dyae078. doi: 10.1093/ije/dyae078. Int J Epidemiol. 2024. PMID: 38840559 Free PMC article.
References
-
- World Health Organization. Timeline: WHO’s COVID-19 response [Internet]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact...
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. [cited 2021 May 15]. Available from: https://covid19.who.int/
-
- American Society for Microbiology. Supply Shortages Impacting COVID-19 and Non-COVID Testing [Internet]. 2020 [cited 2021 May 15]. Available from: https://asm.org/Articles/2020/September/Clinical-Microbiology-Supply-Sho...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

