Comparative performance and cycle threshold values of 10 nucleic acid amplification tests for SARS-CoV-2 on clinical samples
- PMID: 34161355
- PMCID: PMC8221491
- DOI: 10.1371/journal.pone.0252757
Comparative performance and cycle threshold values of 10 nucleic acid amplification tests for SARS-CoV-2 on clinical samples
Erratum in
-
Correction: Comparative performance and cycle threshold values of 10 nucleic acid amplification tests for SARS-CoV-2 on clinical samples.PLoS One. 2024 Nov 27;19(11):e0314897. doi: 10.1371/journal.pone.0314897. eCollection 2024. PLoS One. 2024. PMID: 39602448 Free PMC article.
Abstract
Background: A number of nucleic acid amplification tests (NAATs) for SARS-CoV-2 with different reagents have been approved for clinical use in Japan. These include research kits approved under emergency use authorization through simplified process to stabilize the supply of the reagents. Although these research kits have been increasingly used in clinical practice, limited data is available for the diagnostic performance in clinical settings.
Methods: We compared sensitivity, specificity, and cycle threshold (Ct) values obtained by NAATs using 10 kits approved in Japan including eight kits those receiving emergency use authorization using 69 frozen-stored clinical samples including 23 positive samples with various Ct values and 46 negative samples.
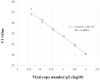
Results: Viral copy number of the frozen-stored samples determined with LightMix E-gene test ranged from 0.6 to 84521.1 copies/μL. While no false-positive results were obtained by any of these tests (specificity: 100% [95% CI, 88.9%-100%]), sensitivity of the nine tests ranged from 68.2% [95% CI, 45.1%-86.1%] to 95.5% [95% CI, 77.2%-99.9%] using LightMix E-gene test as the gold standard. All tests showed positive results for all samples with ≥100 copies/μL. Significant difference of Ct values even among tests amplifying the same genetic region (N1-CDC, N2) was also observed.
Conclusion: Difference in the diagnostic performance was observed among NAATs approved in Japan. Regarding diagnostic kits for emerging infectious diseases, a system is needed to ensure both rapidity of reagent supply and accuracy of diagnosis. Ct values, which are sometimes regarded as a marker of infectivity, are not interchangeable when obtained by different assays.
Conflict of interest statement
Sohei Harada (SH) reports personal fees from BD, Meiji, Shionogi, Sumitomo Dainippon Pharma, MSD, Astellas, Beckman Coulter Diagnostics, FUJIFILM Toyama Chemical, Daiichi Sankyo, and NISSUI PHARMACEUTICAL, and grants from MSD, outside the submitted work. This does not alter our adherence to PLOS ONE policies on sharing data and materials. All other authors declare no conflict of interest.
Figures

Similar articles
-
Comparative evaluation of six nucleic acid amplification kits for SARS-CoV-2 RNA detection.Ann Clin Microbiol Antimicrob. 2021 May 22;20(1):38. doi: 10.1186/s12941-021-00443-w. Ann Clin Microbiol Antimicrob. 2021. PMID: 34022903 Free PMC article.
-
External quality assessment survey for SARS-CoV-2 nucleic acid amplification tests in clinical laboratories in Tokyo, 2021.J Infect Chemother. 2024 Jul;30(7):633-641. doi: 10.1016/j.jiac.2024.01.016. Epub 2024 Feb 5. J Infect Chemother. 2024. PMID: 38325625
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
-
Ensuring accuracy in the development and application of nucleic acid amplification tests (NAATs) for infectious disease.Mol Aspects Med. 2024 Jun;97:101275. doi: 10.1016/j.mam.2024.101275. Epub 2024 May 20. Mol Aspects Med. 2024. PMID: 38772082 Review.
-
A precise review on NAATs-based diagnostic assays for COVID-19: A motion in fast POC molecular tests.Eur J Clin Invest. 2022 Nov;52(11):e13853. doi: 10.1111/eci.13853. Epub 2022 Aug 30. Eur J Clin Invest. 2022. PMID: 35989561 Free PMC article. Review.
Cited by
-
Clinical Performance of the cobas Liat SARS-CoV-2 & Influenza A/B Assay in Nasal Samples.Mol Diagn Ther. 2022 May;26(3):323-331. doi: 10.1007/s40291-022-00580-8. Epub 2022 Apr 7. Mol Diagn Ther. 2022. PMID: 35391608 Free PMC article.
-
Development of a mobile laboratory system in hydrogen fuel cell buses and evaluation of the performance for COVID-19 RT-PCR testing.Sci Rep. 2023 Oct 16;13(1):17546. doi: 10.1038/s41598-023-44925-7. Sci Rep. 2023. PMID: 37845364 Free PMC article.
-
The evaluation of the utility of the GENECUBE HQ SARS-CoV-2 for anterior nasal samples and saliva samples with a new rapid examination protocol.PLoS One. 2021 Dec 31;16(12):e0262159. doi: 10.1371/journal.pone.0262159. eCollection 2021. PLoS One. 2021. PMID: 34972195 Free PMC article.
-
Laboratory Considerations for Reporting Cycle Threshold Value in COVID-19.EJIFCC. 2022 Aug 8;33(2):80-93. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313906 Free PMC article. Review.
-
Association of the Serum Levels of the Nucleocapsid Antigen of SARS-CoV-2 With the Diagnosis, Disease Severity, and Antibody Titers in Patients With COVID-19: A Retrospective Cross-Sectional Study.Front Microbiol. 2021 Dec 9;12:791489. doi: 10.3389/fmicb.2021.791489. eCollection 2021. Front Microbiol. 2021. PMID: 34956158 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

