A genome-wide library of MADM mice for single-cell genetic mosaic analysis
- PMID: 34161767
- PMCID: PMC8317686
- DOI: 10.1016/j.celrep.2021.109274
A genome-wide library of MADM mice for single-cell genetic mosaic analysis
Abstract
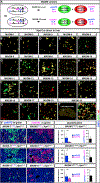
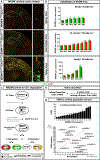
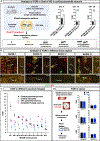
Mosaic analysis with double markers (MADM) offers one approach to visualize and concomitantly manipulate genetically defined cells in mice with single-cell resolution. MADM applications include the analysis of lineage, single-cell morphology and physiology, genomic imprinting phenotypes, and dissection of cell-autonomous gene functions in vivo in health and disease. Yet, MADM can only be applied to <25% of all mouse genes on select chromosomes to date. To overcome this limitation, we generate transgenic mice with knocked-in MADM cassettes near the centromeres of all 19 autosomes and validate their use across organs. With this resource, >96% of the entire mouse genome can now be subjected to single-cell genetic mosaic analysis. Beyond a proof of principle, we apply our MADM library to systematically trace sister chromatid segregation in distinct mitotic cell lineages. We find striking chromosome-specific biases in segregation patterns, reflecting a putative mechanism for the asymmetric segregation of genetic determinants in somatic stem cell division.
Keywords: Mosaic Analysis with Double Markers (MADM); functional gene analysis; genetic mosaic; genomic imprinting; lineage; single cell; sister chromatid Segregation Pattern; stem cell.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Amberg N, Holcmann M, Glitzner E, Novoszel P, Stulnig G, and Sibilia M. (2015). Mouse models of nonmelanoma skin cancer. Methods Mol. Biol 1267, 217–250. - PubMed
-
- Armakolas A, and Klar AJ (2006). Cell type regulates selective segregation of mouse chromosome 7 DNA strands in mitosis. Science 311, 1146–1149. - PubMed
-
- Armakolas A, and Klar AJ (2007). Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science 315, 100–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

