Mesenchymal stem/stromal cell-based therapy for the treatment of rheumatoid arthritis: An update on preclinical studies
- PMID: 34161884
- PMCID: PMC8237294
- DOI: 10.1016/j.ebiom.2021.103427
Mesenchymal stem/stromal cell-based therapy for the treatment of rheumatoid arthritis: An update on preclinical studies
Abstract
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by synovial inflammation and progressive joint destruction and is a primary cause of disability worldwide. Despite the existence of numerous anti-rheumatic drugs, a significant number of patients with RA do not respond or are intolerant to current treatments. Mesenchymal stem/stromal cell (MSCs) therapy represents a promising therapeutic tool to treat RA, mainly attributable to the immunomodulatory effects of these cells. This review comprises a comprehensive analysis of the scientific literature related to preclinical studies of MSC-based therapy in RA to analyse key aspects of current protocols as well as novel approaches which aim to improve the efficacy of MSC-based therapy.
Keywords: Animal models; Improvements in MSC-based therapy; Mesenchymal stem/stromal cells; Protocols; Rheumatoid arthritis.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
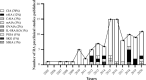

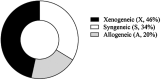
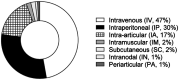

References
-
- Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216655. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

