Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice
- PMID: 34162415
- PMCID: PMC8223331
- DOI: 10.1186/s12974-021-02178-z
Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice
Abstract
Background: Chronic neuropathic pain is a frequent sequel to peripheral nerve injury and maladaptive nervous system function. Divanillyl sulfone (DS), a novel structural derivative of 4,4'-dihydroxydibenzyl sulfoxide from a traditional Chinese medicine Gastrodia elata with anti-nociceptive effects, significantly alleviated neuropathic pain following intrathecal injection. Here, we aimed to investigate the underlying mechanisms of DS against neuropathic pain.
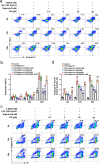
Methods: A chronic constrictive injury (CCI) mouse model of neuropathic pain induced by sciatic nerve ligation was performed to evaluate the effect of DS by measuring the limb withdrawal using Von Frey filament test. Immunofluorescence staining was used to assess the cell localizations and expressions of Iba-1, ASC, NLRP3, and ROS, the formation of autolysosome. The levels of NLRP3-related proteins (caspase-1, NLRP3, and IL-1β), mitophagy-related proteins (LC3, Beclin-1, and p62), and apoptosis-related proteins (Bcl-XL and Bax) were detected by Western blotting. The apoptosis of BV-2 cell and caspase activity were evaluated by flow cytometry.
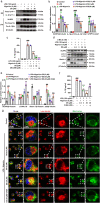
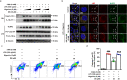
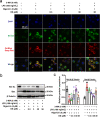
Results: DS significantly alleviated the neuropathic pain by increasing the mechanical withdrawal threshold and inhibiting the activation of NLRP3 in CCI-induced model mice. Our findings indicated that DS promoted the mitophagy by increasing the LC3II and Beclin 1 and decreasing the levels of p62 protein in BV-2 cell. This is accompanied by the inhibition of NLRP3 activation, which was shown as inhibited the expression of NLRP3 in lysates as well as the secretion of mature caspase-1 p10 and IL-1β p17 in supernatants in cultured BV-2 microglia. In addition, DS could promote mitophagy-induced improvement of dysfunctional mitochondria by clearing intracellular ROS and restoring mitochondrial membrane potential.
Conclusion: Together, our findings demonstrated that DS ameliorate chronic neuropathic pain in mice by suppressing NLRP3 inflammasome activation induced by mitophagy in microglia. DS may be a promising therapeutic agent for chronic neuropathic pain.
Keywords: Chronic neuropathic pain; Divanillyl sulfone; Microglia; Mitophagy; NLRP3 inflammasome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

