Clinical evaluation of Sofia Rapid Antigen Assay for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among emergency department to hospital admissions
- PMID: 34162449
- PMCID: PMC8376850
- DOI: 10.1017/ice.2021.281
Clinical evaluation of Sofia Rapid Antigen Assay for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among emergency department to hospital admissions
Abstract
Objective: To determine the utility of the Sofia SARS rapid antigen fluorescent immunoassay (FIA) to guide hospital-bed placement of patients being admitted through the emergency department (ED).
Design: Cross-sectional analysis of a clinical quality improvement study.
Setting: This study was conducted in 2 community hospitals in Maryland from September 21, 2020, to December 3, 2020. In total, 2,887 patients simultaneously received the Sofia SARS rapid antigen FIA and SARS-CoV-2 RT-PCR assays on admission through the ED.
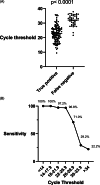
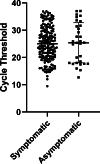
Methods: Rapid antigen results and symptom assessment guided initial patient placement while confirmatory RT-PCR was pending. The sensitivity, specificity, positive predictive values, and negative predictive values of the rapid antigen assay were calculated relative to RT-PCR, overall and separately for symptomatic and asymptomatic patients. Assay sensitivity was compared to RT-PCR cycle threshold (Ct) values. Assay turnaround times were compared. Clinical characteristics of RT-PCR-positive patients and potential exposures from false-negative antigen assays were evaluated.
Results: For all patients, overall agreement was 97.9%; sensitivity was 76.6% (95% confidence interval [CI], 71%-82%), and specificity was 99.7% (95% CI, 99%-100%). We detected no differences in performance between asymptomatic and symptomatic individuals. As RT-PCR Ct increased, the sensitivity of the antigen assay decreased. The mean turnaround time for the antigen assay was 1.2 hours (95% CI, 1.0-1.3) and for RT-PCR it was 20.1 hours (95% CI, 18.9-40.3) (P < .001). No transmission from antigen-negative/RT-PCR-positive patients was identified.
Conclusions: Although not a replacement for RT-PCR for detection of all SARS-CoV-2 infections, the Sofia SARS antigen FIA has clinical utility for potential initial timely patient placement.
Conflict of interest statement
All authors report no conflicts of interest relevant to this article.
Figures
Comment in
-
Utilization of rapid antigen assays for detection of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) in a low-incidence setting in emergency department triage: Does risk-stratification still matter?Infect Control Hosp Epidemiol. 2022 Dec;43(12):1974-1976. doi: 10.1017/ice.2021.407. Epub 2021 Sep 15. Infect Control Hosp Epidemiol. 2022. PMID: 34523394 No abstract available.
References
-
- Guidance document. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised). Immediately in Effect Guidance for Clinical Laboratories, Commercial Manufacturers, and Food and Drug Administration Staff. Food and Drug Administration website. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Published May 2020. Accessed June 23, 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

