Novel enzymatic cross-linking-based hydrogel nanofilm caging system on pancreatic β cell spheroid for long-term blood glucose regulation
- PMID: 34162541
- PMCID: PMC8221614
- DOI: 10.1126/sciadv.abf7832
Novel enzymatic cross-linking-based hydrogel nanofilm caging system on pancreatic β cell spheroid for long-term blood glucose regulation
Abstract
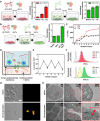
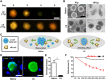
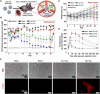
Pancreatic β cell therapy for type 1 diabetes is limited by low cell survival rate owing to physical stress and aggressive host immune response. In this study, we demonstrate a multilayer hydrogel nanofilm caging strategy capable of protecting cells from high shear stress and reducing immune response by interfering cell-cell interaction. Hydrogel nanofilm is fabricated by monophenol-modified glycol chitosan and hyaluronic acid that cross-link each other to form a nanothin hydrogel film on the cell surface via tyrosinase-mediated reactions. Furthermore, hydrogel nanofilm formation was conducted on mouse β cell spheroids for the islet transplantation application. The cytoprotective effect against physical stress and the immune protective effect were evaluated. Last, caged mouse β cell spheroids were transplanted into the type 1 diabetes mouse model and successfully regulated its blood glucose level. Overall, our enzymatic cross-linking-based hydrogel nanofilm caging method will provide a new platform for clinical applications of cell-based therapies.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



Similar articles
-
Transplantation of pancreatic beta-cell spheroids in mice via non-swellable hydrogel microwells composed of poly(HEMA-co-GelMA).Biomater Sci. 2024 Aug 20;12(17):4354-4362. doi: 10.1039/d4bm00295d. Biomater Sci. 2024. PMID: 38967234
-
A bio-inspired injectable hydrogel as a cell platform for real-time glycaemic regulation.J Mater Chem B. 2020 Jun 7;8(21):4627-4641. doi: 10.1039/d0tb00561d. Epub 2020 May 6. J Mater Chem B. 2020. PMID: 32373901
-
Three-dimensional cell-culture platform based on hydrogel with tunable microenvironmental properties to improve insulin-secreting function of MIN6 cells.Biomaterials. 2021 Mar;270:120687. doi: 10.1016/j.biomaterials.2021.120687. Epub 2021 Jan 21. Biomaterials. 2021. PMID: 33540170
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Lessons from Human Islet Transplantation Inform Stem Cell-Based Approaches in the Treatment of Diabetes.Front Endocrinol (Lausanne). 2021 Mar 11;12:636824. doi: 10.3389/fendo.2021.636824. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33776933 Free PMC article. Review.
Cited by
-
Emerging approaches for the development of artificial islets.Smart Med. 2024 Mar 7;3(2):e20230042. doi: 10.1002/SMMD.20230042. eCollection 2024 Jun. Smart Med. 2024. PMID: 39188698 Free PMC article. Review.
-
Cell Surface Engineering Tools for Programming Living Assemblies.Adv Sci (Weinh). 2023 Dec;10(34):e2304040. doi: 10.1002/advs.202304040. Epub 2023 Oct 12. Adv Sci (Weinh). 2023. PMID: 37823678 Free PMC article. Review.
-
Islet Transplantation: Microencapsulation, Nanoencapsulation, and Hypoimmune Engineering.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2025 May-Jun;17(3):e70016. doi: 10.1002/wnan.70016. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2025. PMID: 40394888 Free PMC article. Review.
-
Functional hydrogels for hepatocellular carcinoma: therapy, imaging, and in vitro model.J Nanobiotechnology. 2024 Jul 1;22(1):381. doi: 10.1186/s12951-024-02547-9. J Nanobiotechnology. 2024. PMID: 38951911 Free PMC article. Review.
-
Islet cell spheroids produced by a thermally sensitive scaffold: a new diabetes treatment.J Nanobiotechnology. 2024 Oct 26;22(1):657. doi: 10.1186/s12951-024-02891-w. J Nanobiotechnology. 2024. PMID: 39456025 Free PMC article.
References
-
- Farina M., Alexander J. F., Thekkedath U., Ferrari M., Grattoni A., Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 139, 92–115 (2019). - PubMed
-
- Mitrousis N., Fokina A., Shoichet M. S., Biomaterials for cell transplantation. Nat. Rev. Mater. 3, 441–456 (2018).
-
- Kim H., Shin K., Park O. K., Choi D., Kim H. D., Baik S., Lee S. H., Kwon S. H., Yarema K. J., Hong J., Hyeon T., Hwang N. S., General and facile coating of single cells via mild reduction. J. Am. Chem. Soc. 140, 1199–1202 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

