Electro-steric opening of the CLC-2 chloride channel gate
- PMID: 34162897
- PMCID: PMC8222222
- DOI: 10.1038/s41598-021-92247-3
Electro-steric opening of the CLC-2 chloride channel gate
Abstract
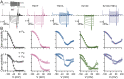
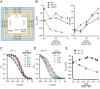
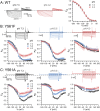
The widely expressed two-pore homodimeric inward rectifier CLC-2 chloride channel regulates transepithelial chloride transport, extracellular chloride homeostasis, and neuronal excitability. Each pore is independently gated at hyperpolarized voltages by a conserved pore glutamate. Presumably, exiting chloride ions push glutamate outwardly while external protonation stabilizes it. To understand the mechanism of mouse CLC-2 opening we used homology modelling-guided structure-function analysis. Structural modelling suggests that glutamate E213 interacts with tyrosine Y561 to close a pore. Accordingly, Y561A and E213D mutants are activated at less hyperpolarized voltages, re-opened at depolarized voltages, and fast and common gating components are reduced. The double mutant cycle analysis showed that E213 and Y561 are energetically coupled to alter CLC-2 gating. In agreement, the anomalous mole fraction behaviour of the voltage dependence, measured by the voltage to induce half-open probability, was strongly altered in these mutants. Finally, cytosolic acidification or high extracellular chloride concentration, conditions that have little or no effect on WT CLC-2, induced reopening of Y561 mutants at positive voltages presumably by the inward opening of E213. We concluded that the CLC-2 gate is formed by Y561-E213 and that outward permeant anions open the gate by electrostatic and steric interactions.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Hille, B., Armstrong, C. M. & MacKinnon, R. Ion channels: From idea to reality. Nat. Med.5, 1105–1109. 10.1038/13415 (1999). - PubMed
-
- Long, S. B., Campbell, E. B. & MacKinnon, R. Voltage sensor of Kv1.2: Structural basis of electromechanical coupling. Science309, 903–908. 10.1126/science.1116270 (2005). - PubMed
-
- Jiang, Y., Ruta, V., Chen, J., Lee, A. & Mackinnon, R. The principle of gating charge movement in a voltage-dependent K+ channel. 423, 42–48 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

