Emergence and spread of a SARS-CoV-2 lineage A variant (A.23.1) with altered spike protein in Uganda
- PMID: 34163035
- PMCID: PMC8318884
- DOI: 10.1038/s41564-021-00933-9
Emergence and spread of a SARS-CoV-2 lineage A variant (A.23.1) with altered spike protein in Uganda
Abstract
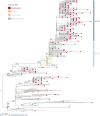
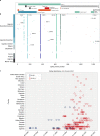
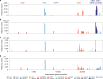
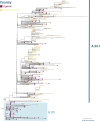
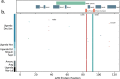
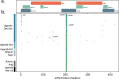
Here, we report SARS-CoV-2 genomic surveillance from March 2020 until January 2021 in Uganda, a landlocked East African country with a population of approximately 40 million people. We report 322 full SARS-CoV-2 genomes from 39,424 reported SARS-CoV-2 infections, thus representing 0.8% of the reported cases. Phylogenetic analyses of these sequences revealed the emergence of lineage A.23.1 from lineage A.23. Lineage A.23.1 represented 88% of the genomes observed in December 2020, then 100% of the genomes observed in January 2021. The A.23.1 lineage was also reported in 26 other countries. Although the precise changes in A.23.1 differ from those reported in the first three SARS-CoV-2 variants of concern (VOCs), the A.23.1 spike-protein-coding region has changes similar to VOCs including a change at position 613, a change in the furin cleavage site that extends the basic amino acid motif and multiple changes in the immunogenic N-terminal domain. In addition, the A.23.1 lineage has changes in non-spike proteins including nsp6, ORF8 and ORF9 that are also altered in other VOCs. The clinical impact of the A.23.1 variant is not yet clear and it has not been designated as a VOC. However, our findings of emergence and spread of this variant indicate that careful monitoring of this variant, together with assessment of the consequences of the spike protein changes for COVID-19 vaccine performance, are advisable.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Holmes, E. C. & Zhang, Y.-Z. Novel 2019 coronavirus genome. Virological.orghttp://virological.org/t/319 (2020).
-
- Volz, E. et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. Preprint at medRxiv10.1101/2020.12.30.20249034 (2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

